Cost-effectiveness of tenecteplase versus alteplase for acute ischemic stroke
- PMID: 37641549
- PMCID: PMC10472948
- DOI: 10.1177/23969873231174943
Cost-effectiveness of tenecteplase versus alteplase for acute ischemic stroke
Abstract
Introduction: Alteplase is widely used as an intravenous thrombolytic drug in acute ischemic stroke (AIS). Recently however, tenecteplase, a modified form of tissue plasminogen activator, has been shown to increase early recanalization rate and has proven to be non-inferior with a similar safety profile compared to alteplase. This study aims to evaluate the cost-effectiveness of 0.25 mg/kg tenecteplase versus 0.9 mg/kg alteplase for intravenous thrombolysis in AIS patients from the Dutch healthcare payer perspective.
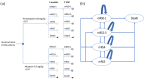
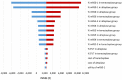
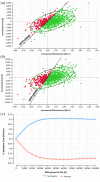
Methods: A Markov decision-analytic model was constructed to assess total costs, total quality-adjusted life year (QALY), an incremental cost-effectiveness ratio, and incremental net monetary benefit (INMB) of two treatments at willingness-to-pay (WTP) thresholds of €50,000/QALY and €80,000/QALY over a 10-year time horizon. One-way sensitivity analysis, probabilistic sensitivity analysis, and scenario analysis were conducted to test the robustness of results. Clinical data were obtained from large randomized controlled trials and real-world data.
Results: Treatment with tenecteplase saved €21 per patient while gaining 0.05 QALYs, resulting in INMB of €2381, clearly rendering tenecteplase cost-effective compared to alteplase. Importantly, tenecteplase remained the cost-effective alternative in all scenarios, including AIS patients due to large vessel occlusion (LVO). Probabilistic sensitivity analysis proved tenecteplase to be cost-effective with a 71.0% probability at a WTP threshold of €50,000/QALY.
Conclusions: Tenecteplase treatment was cost-effective for all AIS patients (including AIS patients with LVO) compared to alteplase. The finding supports the broader use of tenecteplase in acute stroke care, as health outcomes improve at acceptable costs while having practical advantages, and a similar safety profile.
Keywords: Stroke; alteplase; cost-effectiveness; tenecteplase.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Thomalla G, Simonsen CZ, Boutitie F, et al.. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 2018; 379: 611–622. - PubMed
-
- Powers WJ, Rabinstein AA, Ackerson T, et al.. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. - PubMed
-
- European Medicines Agency. Metalyse, https://www.ema.europa.eu/en/medicines/human/EPAR/metalyse (2001, accessed 24 April 2022).
-
- Tanswell P, Modi N, Combs D, et al.. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 2002; 41: 1229–1245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

