Characterizing the emergence of amyloid and tau burden in Down syndrome
- PMID: 37641577
- PMCID: PMC10843570
- DOI: 10.1002/alz.13444
Characterizing the emergence of amyloid and tau burden in Down syndrome
Abstract
Introduction: Almost all individuals with Down syndrome (DS) will develop neuropathological features of Alzheimer's disease (AD). Understanding AD biomarker trajectories is necessary for DS-specific clinical interventions and interpretation of drug-related changes in the disease trajectory.
Methods: A total of 177 adults with DS from the Alzheimer's Biomarker Consortium-Down Syndrome (ABC-DS) underwent positron emission tomography (PET) and MR imaging. Amyloid-beta (Aβ) trajectories were modeled to provide individual-level estimates of Aβ-positive (A+) chronicity, which were compared against longitudinal tau change.
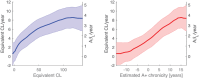
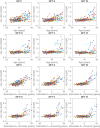
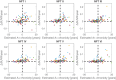
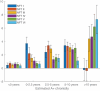
Results: Elevated tau was observed in all NFT regions following A+ and longitudinal tau increased with respect to A+ chronicity. Tau increases in NFT regions I-III was observed 0-2.5 years following A+. Nearly all A+ individuals had tau increases in the medial temporal lobe.
Discussion: These findings highlight the rapid accumulation of amyloid and early onset of tau relative to amyloid in DS and provide a strategy for temporally characterizing AD neuropathology progression that is specific to the DS population and independent of chronological age.
Highlights: Longitudinal amyloid trajectories reveal rapid Aβ accumulation in Down syndrome NFT stage tau was strongly associated with A+ chronicity Early longitudinal tau increases were observed 2.5-5 years after reaching A.
Keywords: Down syndrome; PET; Tau; amyloid; amyloid chronicity; longitudinal; trajectory modeling.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
GE Healthcare holds a license agreement with the University of Pittsburgh based on the technology described in this manuscript. Dr. Klunk is a co‐inventor of PiB and, as such, has a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. AVID Radiopharmaceuticals provided the precursor and reference standard to produce AV‐1451. S.C.J. has served on advisory committees to Roche, Merck, and Alzpath and has received research funding for unrelated work from Cerveau Technologies. All other authors have no conflicts of interest with this work and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors have no conflicts of interest. Author disclosures are available in the supporting information.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG066444/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- U54 HD087011/HD/NICHD NIH HHS/United States
- P50 HD105353/HD/NICHD NIH HHS/United States
- U01 AG051412/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- U54 HD090256/HD/NICHD NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- UL1 TR001857/TR/NCATS NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- U19 AG068054/AG/NIA NIH HHS/United States
- U01 AG051406/AG/NIA NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

