Testicular fat deposition attenuates reproductive performance via decreased follicle-stimulating hormone level and sperm meiosis and testosterone synthesis in mouse
- PMID: 37641828
- PMCID: PMC10766465
- DOI: 10.5713/ab.23.0175
Testicular fat deposition attenuates reproductive performance via decreased follicle-stimulating hormone level and sperm meiosis and testosterone synthesis in mouse
Abstract
Objective: Testicular fat deposition has been reported to affect animal reproduction. However, the underlying mechanism remains poorly understood. The present study explored whether sperm meiosis and testosterone synthesis contribute to mouse testicular fat depositioninduced reproductive performance.
Methods: High fat diet (HFD)-induced obesity CD1 mice (DIO) were used as a testicular fat deposition model. The serum hormone test was performed by agent kit. The quality of sperm was assessed using a Sperm Class Analyzer. Testicular tissue morphology was analyzed by histochemical methods. The expression of spermatocyte marker molecules was monitored by an immuno-fluorescence microscope during meiosis. Analysis of the synthesis of testosterone was performed by real-time polymerase chain reaction and reagent kit.
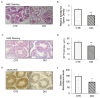
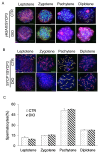
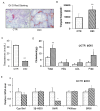
Results: It was found that there was a significant increase in body weight among DIO mice, however, the food intake showed no difference compared to control mice fed a normal diet (CTR). The number of offspring in DIO mice decreased, but there was no significant difference from the CTR group. The levels of follicle-stimulating hormone were lower in DIO mice and their luteinizing hormone levels were similar. The results showed a remarkable decrease in sperm density and motility among DIO mice. We also found that fat accumulation affected the meiosis process, mainly reflected in the cross-exchange of homologous chromosomes. In addition, overweight increased fat deposition in the testis and reduced the expression of testosterone synthesis-related enzymes, thereby affecting the synthesis and secretion of testosterone by testicular Leydig cells.
Conclusion: Fat accumulation in the testes causes testicular cell dysfunction, which affects testosterone hormone synthesis and ultimately affects sperm formation.
Keywords: Cholesterol Metabolism; Overweight; Spermatogenesis; Testosterone.
Conflict of interest statement
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

