TRAF Family Member 4 Promotes Cardiac Hypertrophy Through the Activation of the AKT Pathway
- PMID: 37642020
- PMCID: PMC10547335
- DOI: 10.1161/JAHA.122.028185
TRAF Family Member 4 Promotes Cardiac Hypertrophy Through the Activation of the AKT Pathway
Abstract
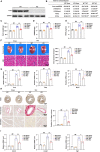
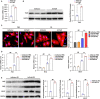
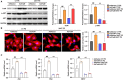
Background Pathological cardiac hypertrophy is a major cause of heart failure morbidity. The complex mechanism of intermolecular interactions underlying the pathogenesis of cardiac hypertrophy has led to a lack of development and application of therapeutic methods. Methods and Results Our study provides the first evidence that TRAF4, a member of the tumor necrosis factor receptor-associated factor (TRAF) family, acts as a promoter of cardiac hypertrophy. Here, Western blotting assays demonstrated that TRAF4 is upregulated in cardiac hypertrophy. Additionally, TRAF4 deletion inhibits the development of cardiac hypertrophy in a mouse model after transverse aortic constriction surgery, whereas its overexpression promotes phenylephrine stimulation-induced cardiomyocyte hypertrophy in primary neonatal rat cardiomyocytes. Mechanistically, RNA-seq analysis revealed that TRAF4 promoted the activation of the protein kinase B pathway during cardiac hypertrophy. Moreover, we found that inhibition of protein kinase B phosphorylation rescued the aggravated cardiomyocyte hypertrophic phenotypes caused by TRAF4 overexpression in phenylephrine-treated neonatal rat cardiomyocytes, suggesting that TRAF4 may regulate cardiac hypertrophy in a protein kinase B-dependent manner. Conclusions Our results revealed the regulatory function of TRAF4 in cardiac hypertrophy, which may provide new insights into developing therapeutic and preventive targets for this disease.
Keywords: AKT pathway; TRAF4; cardiac hypertrophy; heart failure; phenylephrine; primary neonatal rat cardiomyocytes; transverse aortic constriction.
Figures



Similar articles
-
Tripartite motif‑containing 14 may aggravate cardiac hypertrophy via the AKT signalling pathway in neonatal rat cardiomyocytes and transgenic mice.Mol Med Rep. 2023 Sep;28(3):173. doi: 10.3892/mmr.2023.13060. Epub 2023 Jul 28. Mol Med Rep. 2023. PMID: 37503784 Free PMC article.
-
Attenuation of microRNA-16 derepresses the cyclins D1, D2 and E1 to provoke cardiomyocyte hypertrophy.J Cell Mol Med. 2015 Mar;19(3):608-19. doi: 10.1111/jcmm.12445. Epub 2015 Jan 13. J Cell Mol Med. 2015. PMID: 25583328 Free PMC article.
-
Cardiac-specific Traf2 overexpression enhances cardiac hypertrophy through activating AKT/GSK3β signaling.Gene. 2014 Feb 25;536(2):225-31. doi: 10.1016/j.gene.2013.12.052. Epub 2013 Dec 27. Gene. 2014. PMID: 24378234
-
Tumor necrosis factor receptor-associated factor 3 is a positive regulator of pathological cardiac hypertrophy.Hypertension. 2015 Aug;66(2):356-67. doi: 10.1161/HYPERTENSIONAHA.115.05469. Epub 2015 Jun 1. Hypertension. 2015. PMID: 26034202
-
STEAP3 (Six-Transmembrane Epithelial Antigen of Prostate 3) Inhibits Pathological Cardiac Hypertrophy.Hypertension. 2020 Oct;76(4):1219-1230. doi: 10.1161/HYPERTENSIONAHA.120.14752. Epub 2020 Aug 31. Hypertension. 2020. PMID: 32862709
Cited by
-
Extra-osseous Roles of the RANK-RANKL-OPG Axis with a Focus on Skeletal Muscle.Curr Osteoporos Rep. 2024 Dec;22(6):632-650. doi: 10.1007/s11914-024-00890-2. Epub 2024 Sep 26. Curr Osteoporos Rep. 2024. PMID: 39325366 Free PMC article. Review.
-
Tumour necrosis factor receptor-associated factor 7: a new member of the E3 ligase family in cardiac hypertrophy.Cardiovasc Res. 2024 Dec 14;120(16):1987-1988. doi: 10.1093/cvr/cvae225. Cardiovasc Res. 2024. PMID: 39436773 No abstract available.
-
Single-cell RNA sequencing reveals the transcriptional heterogeneity of Tbx18-positive cardiac cells during heart development.Funct Integr Genomics. 2024 Jan 24;24(1):18. doi: 10.1007/s10142-024-01290-6. Funct Integr Genomics. 2024. PMID: 38265516
-
Cardiac Damage in Hypertension: From Molecular Mechanisms to Novel Therapeutic Approaches.Int J Mol Sci. 2025 Jun 11;26(12):5610. doi: 10.3390/ijms26125610. Int J Mol Sci. 2025. PMID: 40565074 Free PMC article. Review.
References
-
- Miao R, Lu Y, Xing X, Li Y, Huang Z, Zhong H, Huang Y, Chen AF, Tang X, Li H, et al. Regulator of G‐protein signaling 10 negatively regulates cardiac remodeling by blocking mitogen‐activated protein kinase‐extracellular signal‐regulated protein kinase 1/2 signaling. Hypertension. 2016;67:86–98. doi: 10.1161/HYPERTENSIONAHA.115.05957 - DOI - PubMed
-
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

