Electrical stimulation alters DNA methylation and promotes neurite outgrowth
- PMID: 37642194
- PMCID: PMC12372594
- DOI: 10.1002/jcb.30462
Electrical stimulation alters DNA methylation and promotes neurite outgrowth
Abstract
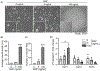
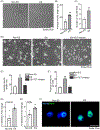
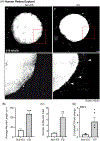
Electrical stimulation (ES) influences neural regeneration and functionality. We here investigate whether ES regulates DNA demethylation, a critical epigenetic event known to influence nerve regeneration. Retinal ganglion cells (RGCs) have long served as a standard model for central nervous system neurons, whose growth and disease development are reportedly affected by DNA methylation. The current study focuses on the ability of ES to rescue RGCs and preserve vision by modulating DNA demethylation. To evaluate DNA demethylation pattern during development, RGCs from mice at different stages of development, were analyzed using qPCR for ten-eleven translocation (TETs) and immunostained for 5 hydroxymethylcytosine (5hmc) and 5 methylcytosine (5mc). To understand the effect of ES on neurite outgrowth and DNA demethylation, cells were subjected to ES at 75 µAmp biphasic ramp for 20 min and cultured for 5 days. ES increased TETs mediated neurite outgrowth, DNA demethylation, TET1 and growth associated protein 43 levels significantly. Immunostaining of PC12 cells following ES for histone 3 lysine 9 trimethylation showed cells attained an antiheterochromatin configuration. Cultured mouse and human retinal explants stained with β-III tubulin exhibited increased neurite growth following ES. Finally, mice subjected to optic nerve crush injury followed by ES exhibited improved RGCs function and phenotype as validated using electroretinogram and immunohistochemistry. Our results point to a possible therapeutic regulation of DNA demethylation by ES in neurons.
Keywords: H3K9Me3; TET1; electrical stimulation; epigenetics; nerve regeneration; retinal ganglion cells.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Dong Chen is scientific founder of FireCyte Therapeutics and a consultant of i-Lumen Scientific and Sichuan PriMed. Kin-Sang Cho is a consultant in FireCyte Therapeutics. Ajay Ashok, Anton Lennikov, Karen Chang, Dong Chen, Kin-Sang Cho, Wai Lydia Tai, and Tor Paas Utheim are inventors of a patent application for using microcurrent electrical stimulation technology or nerve regenerative approach to treat eye diseases.
Figures




References
Grants and funding
- Norwegian Research Council; Department of Ophthalmology, Oslo University Hospital, Oslo, Norway (TU)
- National Eye Institute Grant
- Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway (TU)
- Core Grant for Vision Research from NIH/NEI to the Schepens Eye Research Institute
- R01 EY031696/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources

