Triose phosphate utilization in leaves is modulated by whole-plant sink-source ratios and nitrogen budgets in rice
- PMID: 37642225
- PMCID: PMC10662237
- DOI: 10.1093/jxb/erad329
Triose phosphate utilization in leaves is modulated by whole-plant sink-source ratios and nitrogen budgets in rice
Abstract
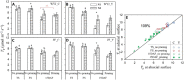
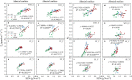
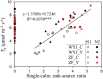
Triose phosphate utilization (TPU) is a biochemical process indicating carbon sink-source (im)balance within leaves. When TPU limits leaf photosynthesis, photorespiration-associated amino acid exports probably provide an additional carbon outlet and increase leaf CO2 uptake. However, whether TPU is modulated by whole-plant sink-source relations and nitrogen (N) budgets remains unclear. We address this question by model analyses of gas-exchange data measured on leaves at three growth stages of rice plants grown at two N levels. Sink-source ratio was manipulated by panicle pruning, by using yellower-leaf variant genotypes, and by measuring photosynthesis on adaxial and abaxial leaf sides. Across all these treatments, higher leaf N content resulted in the occurrence of TPU limitation at lower intercellular CO2 concentrations. Photorespiration-associated amino acid export was greater in high-N leaves, but was smaller in yellower-leaf genotypes, panicle-pruned plants, and for abaxial measurement. The feedback inhibition of panicle pruning on rates of TPU was not always observed, presumably because panicle pruning blocked N remobilization from leaves to grains and the increased leaf N content masked feedback inhibition. The leaf-level TPU limitation was thus modulated by whole-plant sink-source relations and N budgets during rice grain filling, suggesting a close link between within-leaf and whole-plant sink limitations.
Keywords: Oryza sativa; Adaxial versus abaxial measurement; panicle pruning; photorespiration-associated nitrogen assimilation; sink limitation; triose phosphate utilization; yellower-leaf modification.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Is triose phosphate utilization involved in the feedback inhibition of photosynthesis in rice under conditions of sink limitation?J Exp Bot. 2019 Oct 24;70(20):5773-5785. doi: 10.1093/jxb/erz318. J Exp Bot. 2019. PMID: 31269202
-
Genotypic variation in source and sink traits affects the response of photosynthesis and growth to elevated atmospheric CO2.Plant Cell Environ. 2020 Mar;43(3):579-593. doi: 10.1111/pce.13693. Epub 2020 Jan 21. Plant Cell Environ. 2020. PMID: 31961455
-
Triose phosphate utilization and beyond: from photosynthesis to end product synthesis.J Exp Bot. 2019 Mar 27;70(6):1755-1766. doi: 10.1093/jxb/erz058. J Exp Bot. 2019. PMID: 30868155 Free PMC article. Review.
-
Short-term salt stress reduces photosynthetic oscillations under triose phosphate utilization limitation in tomato.J Exp Bot. 2024 May 20;75(10):2994-3008. doi: 10.1093/jxb/erae089. J Exp Bot. 2024. PMID: 38436737
-
Increasing rice productivity and yield by manipulation of starch synthesis.Novartis Found Symp. 2001;236:135-46; discussion 147-52. doi: 10.1002/9780470515778.ch10. Novartis Found Symp. 2001. PMID: 11387976 Review.
References
-
- Abadie C, Boex-Fontvieille ERA, Carroll AJ, Tcherkez G.. 2016. In vivo stoichiometry of photorespiratory metabolism. Nature Plants 2, 15220. - PubMed
-
- Arp WJ. 1991. Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant, Cell & Environment 14, 869–875.
-
- Bauwe H, Hagemann M, Fernie AR.. 2010. Photorespiration: players, partners and origin. Trends in Plant Science 15, 330–336. - PubMed
-
- Bloom AJ, Asensio JSR, Randall L, Rachmilevitch S, Cousins AB, Carlisle EA.. 2012. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 93, 355–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

