Effects of dipeptidyl peptidase 4 inhibition on the endothelial control of the vascular tone
- PMID: 37642237
- PMCID: PMC11932530
- DOI: 10.1152/ajpcell.00246.2023
Effects of dipeptidyl peptidase 4 inhibition on the endothelial control of the vascular tone
Abstract
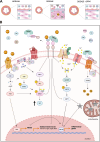
Dipeptidyl peptidase 4 (DPP4) is a serine protease known to cleave incretin hormones, which stimulate insulin secretion after food intake, a fact that supported the development of its inhibitors (DPP4i or gliptins) for the treatment of type 2 diabetes mellitus. In addition to their glucose-lowering effects, DPP4i show benefits for the cardiovascular system that could be related, at least in part, to their protective action on vascular endothelium. DPP4i have been associated with the reversal of endothelial dysfunction, an important predictor of cardiovascular events and a hallmark of diseases such as atherosclerosis, diabetes mellitus, hypertension, and heart failure. In animal models of these diseases, DPP4i increase nitric oxide bioavailability and limits oxidative stress, thereby improving the endothelium-dependent relaxation. Similar effects on flow-mediated dilation and attenuation of endothelial dysfunction have also been noted in human studies, suggesting a value for gliptins in the clinical scenario, despite the variability of the results regarding the DPP4i used, treatment duration, and presence of comorbidities. In this mini-review, we discuss the advances in our comprehension of the DPP4i effects on endothelial regulation of vascular tone. Understanding the role of DPP4 and its involvement in the signaling mechanisms leading to endothelial dysfunction will pave the way for a broader use of DPP4i in conditions that endothelial dysfunction is a pivotal pathophysiological player.
Keywords: DPP-IV; cardiovascular diseases; eNOS; gliptins; reactive oxygen species.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Role of DPP4 and DPP4i in Glucose Homeostasis and Cardiorenal Syndrome.Endocr Metab Immune Disord Drug Targets. 2023;23(2):179-187. doi: 10.2174/1871530322666220531123116. Endocr Metab Immune Disord Drug Targets. 2023. PMID: 35642117 Review.
-
Recent Advances in Dipeptidyl-Peptidase-4 Inhibition Therapy: Lessons from the Bench and Clinical Trials.J Diabetes Res. 2015;2015:606031. doi: 10.1155/2015/606031. Epub 2015 May 14. J Diabetes Res. 2015. PMID: 26075284 Free PMC article. Review.
-
Cardiovascular protection by DPP-4 inhibitors in preclinical studies: an updated review of molecular mechanisms.Naunyn Schmiedebergs Arch Pharmacol. 2022 Nov;395(11):1357-1372. doi: 10.1007/s00210-022-02279-3. Epub 2022 Aug 10. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35945358 Free PMC article. Review.
-
Cardiovascular pleiotropic actions of DPP-4 inhibitors: a step at the cutting edge in understanding their additional therapeutic potentials.Cell Signal. 2013 Sep;25(9):1799-803. doi: 10.1016/j.cellsig.2013.05.009. Epub 2013 May 22. Cell Signal. 2013. PMID: 23707531 Review.
-
Vascular protection with dipeptidyl peptidase-IV inhibitors in diabetes: experimental and clinical therapeutics.Pharmacotherapy. 2015 Mar;35(3):277-97. doi: 10.1002/phar.1547. Epub 2015 Mar 10. Pharmacotherapy. 2015. PMID: 25754657 Review.
Cited by
-
Assessing the benefit-risk profile of newer glucose-lowering drugs: A systematic review and network meta-analysis of randomized outcome trials.Diabetes Obes Metab. 2025 Mar;27(3):1444-1455. doi: 10.1111/dom.16147. Epub 2024 Dec 26. Diabetes Obes Metab. 2025. PMID: 39723481
-
The impact of DPP-4 inhibitors on cardiovascular disease treatment: a comprehensive review of current therapeutic strategies and future directions.Mol Biol Rep. 2025 Apr 17;52(1):400. doi: 10.1007/s11033-025-10458-7. Mol Biol Rep. 2025. PMID: 40244362 Review.
-
Synthetic Approaches to Novel DPP-IV Inhibitors-A Literature Review.Molecules. 2025 Feb 25;30(5):1043. doi: 10.3390/molecules30051043. Molecules. 2025. PMID: 40076268 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

