Polar Heterobenzylic C(sp3)-H Chlorination Pathway Enabling Efficient Diversification of Aromatic Nitrogen Heterocycles
- PMID: 37642292
- PMCID: PMC10629438
- DOI: 10.1021/jacs.3c05822
Polar Heterobenzylic C(sp3)-H Chlorination Pathway Enabling Efficient Diversification of Aromatic Nitrogen Heterocycles
Abstract
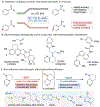
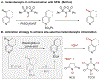
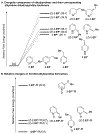
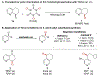
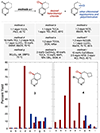
Site-selective radical reactions of benzylic C-H bonds are now highly effective methods for C(sp3-H) functionalization and cross-coupling. The existing methods, however, are often ineffective with heterobenzylic C-H bonds in alkyl-substituted pyridines and related aromatic heterocycles that are prominently featured in pharmaceuticals and agrochemicals. Here, we report new synthetic methods that leverage polar, rather than radical, reaction pathways to enable the selective heterobenzylic C-H chlorination of 2- and 4-alkyl-substituted pyridines and other heterocycles. Catalytic activation of the substrate with trifluoromethanesulfonyl chloride promotes the formation of enamine tautomers that react readily with electrophilic chlorination reagents. The resulting heterobenzyl chlorides can be used without isolation or purification in nucleophilic coupling reactions. This chlorination-diversification sequence provides an efficient strategy to achieve heterobenzylic C-H cross-coupling with aliphatic amines and a diverse collection of azoles, among other coupling partners.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-like Molecules. Chem. Soc. Rev 2016, 45, 546–576. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

