Direct Synthesis of Formamide from CO2 and H2O with Nickel-Iron Nitride Heterostructures under Mild Hydrothermal Conditions
- PMID: 37642297
- PMCID: PMC7615090
- DOI: 10.1021/jacs.3c05412
Direct Synthesis of Formamide from CO2 and H2O with Nickel-Iron Nitride Heterostructures under Mild Hydrothermal Conditions
Abstract
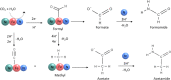
Formamide can serve as a key building block for the synthesis of organic molecules relevant to premetabolic processes. Natural pathways for its synthesis from CO2 under early earth conditions are lacking. Here, we report the thermocatalytic conversion of CO2 and H2O to formate and formamide over Ni-Fe nitride heterostructures in the absence of synthetic H2 and N2 under mild hydrothermal conditions. While water molecules act as both a solvent and hydrogen source, metal nitrides serve as nitrogen sources to produce formamide in the temperature range of 25-100 °C under 5-50 bar. Longer reaction times promote the C-C bond coupling and formation of acetate and acetamide as additional products. Besides liquid products, methane and ethane are also produced as gas-phase products. Postreaction characterization of Ni-Fe nitride particles reveals structural alteration and provides insights into the potential reaction mechanism. The findings indicate that gaseous CO2 can serve as a carbon source for the formation of C-N bonds in formamide and acetamide over the Ni-Fe nitride heterostructure under simulated hydrothermal vent conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
CO2 Fixation to Prebiotic Intermediates over Heterogeneous Catalysts.Acc Chem Res. 2024 Aug 6;57(15):2038-2047. doi: 10.1021/acs.accounts.4c00151. Epub 2024 Jul 18. Acc Chem Res. 2024. PMID: 39024180 Free PMC article.
-
Influence of Composition of Nickel-Iron Nanoparticles for Abiotic CO2 Conversion to Early Prebiotic Organics.Angew Chem Int Ed Engl. 2023 May 22;62(22):e202218189. doi: 10.1002/anie.202218189. Epub 2023 Apr 19. Angew Chem Int Ed Engl. 2023. PMID: 36951652
-
Chemical Antiquity in Metabolism.Acc Chem Res. 2024 Aug 20;57(16):2267-2278. doi: 10.1021/acs.accounts.4c00226. Epub 2024 Jul 31. Acc Chem Res. 2024. PMID: 39083571 Free PMC article.
-
All-in-One CO2 Capture and Transformation: Lessons from Formylmethanofuran Dehydrogenases.Acc Chem Res. 2024 Dec 17;57(24):3512-3523. doi: 10.1021/acs.accounts.4c00623. Epub 2024 Nov 25. Acc Chem Res. 2024. PMID: 39584476 Free PMC article. Review.
-
Hydrothermal Reduction of CO2 to Value-Added Products by In Situ Generated Metal Hydrides.Materials (Basel). 2023 Apr 6;16(7):2902. doi: 10.3390/ma16072902. Materials (Basel). 2023. PMID: 37049198 Free PMC article. Review.
Cited by
-
CO2 Fixation to Prebiotic Intermediates over Heterogeneous Catalysts.Acc Chem Res. 2024 Aug 6;57(15):2038-2047. doi: 10.1021/acs.accounts.4c00151. Epub 2024 Jul 18. Acc Chem Res. 2024. PMID: 39024180 Free PMC article.
-
Revisiting Pt foil catalysts for formamide electrosynthesis achieved at industrial-level current densities.Nat Commun. 2025 Aug 28;16(1):8040. doi: 10.1038/s41467-025-63313-5. Nat Commun. 2025. PMID: 40877263 Free PMC article.
-
Photocatalytic C-N coupling from stable and transient intermediates for gram-scale acetamide synthesis.Nat Commun. 2025 Apr 15;16(1):3590. doi: 10.1038/s41467-025-58840-0. Nat Commun. 2025. PMID: 40234397 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

