Saturating growth rate against phosphorus concentration explained by macromolecular allocation
- PMID: 37642424
- PMCID: PMC10654069
- DOI: 10.1128/msystems.00611-23
Saturating growth rate against phosphorus concentration explained by macromolecular allocation
Abstract
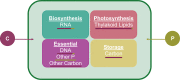
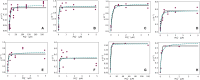
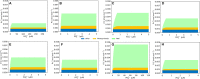
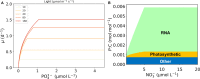
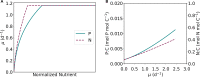
The Monod equation has been used to represent the relationship between growth rate and the environmental nutrient concentration under the limitation of this respective nutrient. This model often serves as a means to connect microorganisms to their environment, specifically in ecosystem and global models. Here, we use a simple model of a marine microorganism cell to illustrate the model's ability to capture the same relationship as Monod, while highlighting the additional physiological details our model provides. In this study, we focus on the relationship between growth rate and phosphorus concentration and find that RNA allocation largely contributes to the commonly observed trend. This work emphasizes the potential role our model could play in connecting microorganisms to the surrounding environment while using realistic physiological representations.
Keywords: DNA; Monod kinetics; RNA; carbohydrate; growth; lipid; macromolecular allocation; nutrient; nutrient storage; phytoplankton; protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hülse D, Arndt S, Wilson JD, Munhoven G, Ridgwell A. 2017. Understanding the causes and consequences of past marine carbon cycling variability through models. Earth Sci Rev 171:349–382. doi: 10.1016/j.earscirev.2017.06.004 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

