Isolation, biochemical characterization, and genome sequencing of two high-quality genomes of a novel chitinolytic Jeongeupia species
- PMID: 37642486
- PMCID: PMC10404844
- DOI: 10.1002/mbo3.1372
Isolation, biochemical characterization, and genome sequencing of two high-quality genomes of a novel chitinolytic Jeongeupia species
Abstract
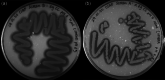
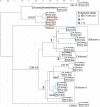
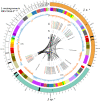
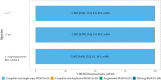
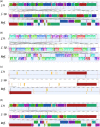
Chitin is the second most abundant polysaccharide worldwide as part of arthropods' exoskeletons and fungal cell walls. Low concentrations in soils and sediments indicate rapid decomposition through chitinolytic organisms in terrestrial and aquatic ecosystems. The enacting enzymes, so-called chitinases, and their products, chitooligosaccharides, exhibit promising characteristics with applications ranging from crop protection to cosmetics, medical, textile, and wastewater industries. Exploring novel chitinolytic organisms is crucial to expand the enzymatical toolkit for biotechnological chitin utilization and to deepen our understanding of diverse catalytic mechanisms. In this study, we present two long-read sequencing-based genomes of highly similar Jeongeupia species, which have been screened, isolated, and biochemically characterized from chitin-amended soil samples. Through metabolic characterization, whole-genome alignments, and phylogenetic analysis, we could demonstrate how the investigated strains differ from the taxonomically closest strain Jeongeupia naejangsanensis BIO-TAS4-2T (DSM 24253). In silico analysis and sequence alignment revealed a multitude of highly conserved chitinolytic enzymes in the investigated Jeongeupia genomes. Based on these results, we suggest that the two strains represent a novel species within the genus of Jeongeupia, which may be useful for environmentally friendly N-acetylglucosamine production from crustacean shell or fungal biomass waste or as a crop protection agent.
Keywords: Neisseria; bioinformatics; comparative genomics; taxonomy.
© 2023 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Proteomic and Transcriptomic Analyses to Decipher the Chitinolytic Response of Jeongeupia spp.Mar Drugs. 2023 Aug 15;21(8):448. doi: 10.3390/md21080448. Mar Drugs. 2023. PMID: 37623729 Free PMC article.
-
Jeongeupia chitinilytica sp. nov., a chitinolytic bacterium isolated from soil.Int J Syst Evol Microbiol. 2013 Mar;63(Pt 3):934-938. doi: 10.1099/ijs.0.043125-0. Epub 2012 Jun 1. Int J Syst Evol Microbiol. 2013. PMID: 22659500
-
Genome Analysis of Fimbriiglobus ruber SP5T, a Planctomycete with Confirmed Chitinolytic Capability.Appl Environ Microbiol. 2018 Mar 19;84(7):e02645-17. doi: 10.1128/AEM.02645-17. Print 2018 Apr 1. Appl Environ Microbiol. 2018. PMID: 29374042 Free PMC article.
-
Recent advances in the bioprospection and applications of chitinolytic bacteria for valorization of waste chitin.Arch Microbiol. 2021 Jul;203(5):1953-1969. doi: 10.1007/s00203-021-02234-5. Epub 2021 Mar 12. Arch Microbiol. 2021. PMID: 33710379 Review.
-
Biotechnological aspects of chitinolytic enzymes: a review.Appl Microbiol Biotechnol. 2006 Aug;71(6):773-82. doi: 10.1007/s00253-005-0183-7. Epub 2006 Jul 21. Appl Microbiol Biotechnol. 2006. PMID: 16249876 Review.
Cited by
-
Proteomic and Transcriptomic Analyses to Decipher the Chitinolytic Response of Jeongeupia spp.Mar Drugs. 2023 Aug 15;21(8):448. doi: 10.3390/md21080448. Mar Drugs. 2023. PMID: 37623729 Free PMC article.
-
High Degree of Polymerization of Chitin Oligosaccharides Produced from Shrimp Shell Waste by Enrichment Microbiota Using Two-Stage Temperature-Controlled Technique of Inducing Enzyme Production and Metagenomic Analysis of Microbiota Succession.Mar Drugs. 2024 Jul 28;22(8):346. doi: 10.3390/md22080346. Mar Drugs. 2024. PMID: 39195462 Free PMC article.
References
-
- Abu Hassan, M. A. , Tan, P. L. , & Zainon Noor, Z. (2009). Coagulation and flocculation treatment of wastewater in textile industry using chitosan. Journal of Chemical and Natural Resources Engineering, 4, 43–53.
-
- Afgan, E. , Baker, D. , van den Beek, M. , Blankenberg, D. , Bouvier, D. , Čech, M. , Chilton, J. , Clements, D. , Coraor, N. , Eberhard, C. , Grüning, B. , Guerler, A. , Hillman‐Jackson, J. , Von Kuster, G. , Rasche, E. , Soranzo, N. , Turaga, N. , Taylor, J. , Nekrutenko, A. , & Goecks, J. (2016). The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Research, 44, W3–W10. 10.1093/nar/gkw343 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

