Evaluating models and assessment techniques for understanding oral biofilm complexity
- PMID: 37642488
- PMCID: PMC10464519
- DOI: 10.1002/mbo3.1377
Evaluating models and assessment techniques for understanding oral biofilm complexity
Abstract
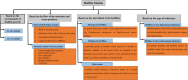
Oral biofilms are three-dimensional (3D) complex entities initiating dental diseases and have been evaluated extensively in the scientific literature using several biofilm models and assessment techniques. The list of biofilm models and assessment techniques may overwhelm a novice biofilm researcher. This narrative review aims to summarize the existing literature on biofilm models and assessment techniques, providing additional information on selecting an appropriate model and corresponding assessment techniques, which may be useful as a guide to the beginner biofilm investigator and as a refresher to experienced researchers. The review addresses previously established 2D models, outlining their advantages and limitations based on the growth environment, availability of nutrients, and the number of bacterial species, while also exploring novel 3D biofilm models. The growth of biofilms on clinically relevant 3D models, particularly melt electrowritten fibrous scaffolds, is discussed with a specific focus that has not been previously reported. Relevant studies on validated oral microcosm models that have recently gaining prominence are summarized. The review analyses the advantages and limitations of biofilm assessment methods, including colony forming unit culture, crystal violet, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt assays, confocal microscopy, fluorescence in situ hybridization, scanning electron microscopy, quantitative polymerase chain reaction, and next-generation sequencing. The use of more complex models with advanced assessment methodologies, subject to the availability of equipment/facilities, may help in developing clinically relevant biofilms and answering appropriate research questions.
Keywords: assessment; biofilm; dynamic; polymicrobial; three-dimensional model.
© 2023 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures






Similar articles
-
Fabrication and characterization of a 3D polymicrobial microcosm biofilm model using melt electrowritten scaffolds.Biomater Adv. 2023 Feb;145:213251. doi: 10.1016/j.bioadv.2022.213251. Epub 2022 Dec 21. Biomater Adv. 2023. PMID: 36580768
-
Effect of batch and fed-batch growth modes on biofilm formation by Listeria monocytogenes at different temperatures.Curr Microbiol. 2009 Oct;59(4):457-62. doi: 10.1007/s00284-009-9460-5. Epub 2009 Aug 4. Curr Microbiol. 2009. PMID: 19653035
-
Quantifying implant-associated biofilms: Comparison of microscopic, microbiologic and biochemical methods.J Microbiol Methods. 2016 Nov;130:61-68. doi: 10.1016/j.mimet.2016.07.016. Epub 2016 Jul 19. J Microbiol Methods. 2016. PMID: 27444546
-
How Biofilms Evade Host Defenses.Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MB-0012-2014. Microbiol Spectr. 2015. PMID: 26185085 Review.
-
Imaging biofilms using fluorescence in situ hybridization: seeing is believing.Front Cell Infect Microbiol. 2023 May 22;13:1195803. doi: 10.3389/fcimb.2023.1195803. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37284501 Free PMC article. Review.
Cited by
-
Short-Term Probiotic Colonization Alters Molecular Dynamics of 3D Oral Biofilms.Int J Mol Sci. 2025 Jul 3;26(13):6403. doi: 10.3390/ijms26136403. Int J Mol Sci. 2025. PMID: 40650181 Free PMC article.
-
Biofilm Formation on Hybrid, Resin-Based CAD/CAM Materials for Indirect Restorations: A Comprehensive Review.Materials (Basel). 2024 Mar 23;17(7):1474. doi: 10.3390/ma17071474. Materials (Basel). 2024. PMID: 38611989 Free PMC article. Review.
-
Reduced Biofilm Accumulation on Implants Treated With Implantoplasty-An In Situ Trial With a Within-Subject Comparison.Clin Exp Dent Res. 2024 Dec;10(6):e70043. doi: 10.1002/cre2.70043. Clin Exp Dent Res. 2024. PMID: 39610010 Free PMC article. Clinical Trial.
-
Rhamnolipid 89 Biosurfactant Is Effective against Streptococcus oralis Biofilm and Preserves Osteoblast Behavior: Perspectives in Dental Implantology.Int J Mol Sci. 2023 Sep 13;24(18):14014. doi: 10.3390/ijms241814014. Int J Mol Sci. 2023. PMID: 37762317 Free PMC article.
-
Viable but non-cultivable state in oral microbiota: a critical review of an underexplored microbial survival strategy.Front Cell Infect Microbiol. 2025 Mar 18;15:1533768. doi: 10.3389/fcimb.2025.1533768. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40171166 Free PMC article. Review.
References
-
- Abdal‐hay, A. , Ramachandra, S. S. , Q. Alali, A. , Han, P. , Sheikh, F. A. , Hashem, M. , & Ivanovski, S. (2023). Vertically aligned calcium phosphate nanoplates coated onto melt electrowritten 3D poly(ε‐caprolactone) fibrous scaffolds for inhibiting biofilm formation. Journal of Alloys and Compounds, 966, 171565.
-
- Abdo, V. L. , Suarez, L. J. , de Paula, L. G. , Costa, R. C. , Shibli, J. , Feres, M. , Barão, V. A. R. , Bertolini, M. , & Souza, J. G. S. (2023). Underestimated microbial infection of resorbable membranes on guided regeneration. Colloids and Surfaces B: Biointerfaces, 226, 113318. - PubMed
-
- Arciola, C. R. , Campoccia, D. , & Montanaro, L. (2018). Implant infections: Adhesion, biofilm formation and immune evasion. Nature Reviews Microbiology, 16(7), 397–409. - PubMed
-
- ATCC . (2020). ATCC: The Global Bioresource Center. https://www.atcc.org/
-
- Azeredo, J. , Azevedo, N. F. , Briandet, R. , Cerca, N. , Coenye, T. , Costa, A. R. , Desvaux, M. , Di Bonaventura, G. , Hébraud, M. , Jaglic, Z. , Kačániová, M. , Knøchel, S. , Lourenço, A. , Mergulhão, F. , Meyer, R. L. , Nychas, G. , Simões, M. , Tresse, O. , & Sternberg, C. (2017). Critical review on biofilm methods. Critical Reviews in Microbiology, 43(3), 313–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

