Phosphorylation-state dependent intraneuronal sorting of Aβ differentially impairs autophagy and the endo-lysosomal system
- PMID: 37642583
- PMCID: PMC10761119
- DOI: 10.1080/15548627.2023.2252300
Phosphorylation-state dependent intraneuronal sorting of Aβ differentially impairs autophagy and the endo-lysosomal system
Abstract
AD: Alzheimer disease; APP: amyloid beta precursor protein; ATG: autophagy related; Aβ: amyloid-β; CTSD: cathepsin D; DAPI: 4',6-diamidino-2-phenylindole; EEA1: early endosome antigen 1; FA: formic acid; GFP: green fluorescent protein; LAMP2: lysosomal-associated membrane protein 2; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; MAP2: microtubule-associated protein 2; nmAβ: non-modified amyloid-β; npAβ: non-phosphorylated amyloid-β; pAβ: phosphorylated amyloid-β; p-Ser26Aβ: amyloid-β phosphorylated at serine residue 26; p-Ser8Aβ: amyloid-β phosphorylated at serine residue 8; RAB: RAB, member RAS oncogene family; RFP: red fluorescent protein; SQSTM1/p62: sequestome 1; YFP: yellow fluorescent protein.
Keywords: Alzheimer’s disease; autophagic flux; neurodegeneration; phosphorylated Aβ; post-translationally modified Aβ; vesicular trafficking.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
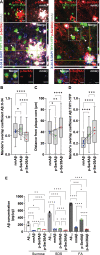
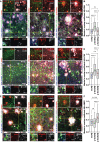
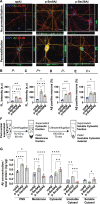
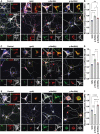
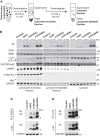
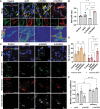
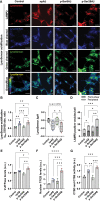

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
