Early-life exposure to cigarette smoke primes lung function and DNA methylation changes at Cyp1a1 upon exposure later in life
- PMID: 37642652
- PMCID: PMC11068412
- DOI: 10.1152/ajplung.00192.2023
Early-life exposure to cigarette smoke primes lung function and DNA methylation changes at Cyp1a1 upon exposure later in life
Abstract
Prenatal and early-life exposure to cigarette smoke (CS) has repeatedly been shown to induce stable, long-term changes in DNA methylation (DNAm) in offspring. It has been hypothesized that these changes might be functionally related to the known outcomes of prenatal and early-life CS exposure, which include impaired lung development, altered lung function, and increased risk of asthma and wheeze. However, to date, few studies have examined DNAm changes induced by prenatal CS in tissues of the lung, and even fewer have attempted to examine the specific influences of prenatal versus early postnatal exposures. Here, we have established a mouse model of CS exposure which isolates the effects of prenatal and early postnatal CS exposures in early life. We have used this model to measure the effects of prenatal and/or postnatal CS exposures on lung function and immune cell infiltration as well as DNAm and expression of Cyp1a1, a candidate gene previously observed to demonstrate DNAm differences on CS exposure in humans. Our study revealed that exposure to CS prenatally and in the early postnatal period causes long-lasting differences in offspring lung function, gene expression, and lung Cyp1a1 DNAm, which wane over time but are reestablished on reexposure to CS in adulthood. This study creates a testable mouse model that can be used to investigate the effects of prenatal and early postnatal CS exposures and will contribute to the design of intervention strategies to mediate these detrimental effects.NEW & NOTEWORTHY Here, we isolated effects of prenatal from early postnatal cigarette smoke and showed that exposure to cigarette smoke early in life causes changes in offspring DNA methylation at Cyp1a1 that last through early adulthood but not into late adulthood. We also showed that smoking in adulthood reestablished these DNA methylation patterns at Cyp1a1, suggesting that a mechanism other than DNA methylation results in long-term memory associated with early-life cigarette smoke exposures at this gene.
Keywords: DNA methylation; cigarette smoke; early life; lung function; priming.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

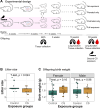
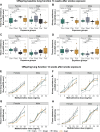





Similar articles
-
Prenatal and Postnatal Cigarette Smoke Exposure Is Associated With Increased Risk of Exacerbated Allergic Airway Immune Responses: A Preclinical Mouse Model.Front Immunol. 2021 Dec 23;12:797376. doi: 10.3389/fimmu.2021.797376. eCollection 2021. Front Immunol. 2021. PMID: 35003121 Free PMC article.
-
In Utero Cigarette Smoke Affects Allergic Airway Disease But Does Not Alter the Lung Methylome.PLoS One. 2015 Dec 7;10(12):e0144087. doi: 10.1371/journal.pone.0144087. eCollection 2015. PLoS One. 2015. PMID: 26642056 Free PMC article.
-
Changes in first trimester fetal CYP1A1 and AHRR DNA methylation and mRNA expression in response to exposure to maternal cigarette smoking.Environ Toxicol Pharmacol. 2018 Jan;57:19-27. doi: 10.1016/j.etap.2017.11.007. Epub 2017 Nov 16. Environ Toxicol Pharmacol. 2018. PMID: 29169084
-
A systematic review of smoking-related epigenetic alterations.Arch Toxicol. 2019 Oct;93(10):2715-2740. doi: 10.1007/s00204-019-02562-y. Epub 2019 Sep 25. Arch Toxicol. 2019. PMID: 31555878
-
DNA methylation alterations in response to prenatal exposure of maternal cigarette smoking: A persistent epigenetic impact on health from maternal lifestyle?Arch Toxicol. 2016 Feb;90(2):231-45. doi: 10.1007/s00204-014-1426-0. Epub 2014 Dec 6. Arch Toxicol. 2016. PMID: 25480659 Review.
Cited by
-
Novel DNA methylation changes in mouse lungs associated with chronic smoking.Epigenetics. 2024 Dec;19(1):2322386. doi: 10.1080/15592294.2024.2322386. Epub 2024 Mar 4. Epigenetics. 2024. PMID: 38436597 Free PMC article.
-
Early career member highlights from the 22nd ERS Lung Science Conference: development of chronic lung diseases - from life-spanning mechanisms to preventive therapy.ERJ Open Res. 2024 Dec 16;10(6):00659-2024. doi: 10.1183/23120541.00659-2024. eCollection 2024 Nov. ERJ Open Res. 2024. PMID: 39687388 Free PMC article.
References
-
- Statistics Canada. Smokers, by Age Group (Online). https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009610&pickMem... [2019 Apr 19].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

