Computational modeling based on confocal imaging predicts changes in osteocyte and dendrite shear stress due to canalicular loss with aging
- PMID: 37642807
- PMCID: PMC12183637
- DOI: 10.1007/s10237-023-01763-w
Computational modeling based on confocal imaging predicts changes in osteocyte and dendrite shear stress due to canalicular loss with aging
Abstract
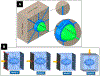
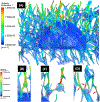
Exercise and physical activity exert mechanical loading on the bones which induces bone formation. However, the relationship between the osteocyte lacunar-canalicular morphology and mechanical stress experienced locally by osteocytes transducing signals for bone formation is not fully understood. In this study, we used computational modeling to predict the effect of canalicular density, the number of fluid inlets, and load direction on fluid flow shear stress (FFSS) and bone strains and how these might change following the microstructural deterioration of the lacunar-canalicular network that occurs with aging. Four distinct computational models were initially generated of osteocytes with either ten or eighteen dendrites using a fluid-structure interaction method with idealized geometries. Next, a young and a simulated aged osteocyte were developed from confocal images after FITC staining of the femur of a 4-month-old C57BL/6 mouse to estimate FFSS using a computational fluid dynamics approach. The models predicted higher fluid velocities in the canaliculi versus the lacunae. Comparison of idealized models with five versus one fluid inlet indicated that with four more inlets, one-half of the dendrites experienced FFSS greater than 0.8 Pa, which has been associated with osteogenic responses. Confocal image-based models of real osteocytes indicated a six times higher ratio of canalicular to lacunar surface area in the young osteocyte model than the simulated aged model and the average FFSS in the young model (FFSS = 0.46 Pa) was three times greater than the aged model (FFSS = 0.15 Pa). Interestingly, the surface area with FFSS values above 0.8 Pa was 23 times greater in the young versus the simulated aged model. These findings may explain the impaired mechano-responsiveness of osteocytes with aging.
Keywords: Aging; Computational fluid dynamics (CFD); Confocal imaging; Fluid–structure interaction (FSI); Mechanotransduction; Osteocyte.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Declarations
Figures


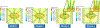


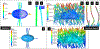
Similar articles
-
Analysis and Imaging of Osteocytes.J Vis Exp. 2024 Nov 29;(213):10.3791/64699. doi: 10.3791/64699. J Vis Exp. 2024. PMID: 39671327
-
MLO-Y4 fluid flow shear stress conditioned media enhances cardiac contractility and intracellular Ca2.Am J Physiol Regul Integr Comp Physiol. 2025 May 1;328(5):R591-R600. doi: 10.1152/ajpregu.00287.2024. Epub 2025 Mar 26. Am J Physiol Regul Integr Comp Physiol. 2025. PMID: 40135808 Free PMC article.
-
Osteocyte-lacuna shape and canaliculi architecture dictate fluid flow around osteocyte, and strain of cell and bone matrix: implications for cell mechanobiology and bone fragility.Bone. 2025 Aug 22;200:117613. doi: 10.1016/j.bone.2025.117613. Online ahead of print. Bone. 2025. PMID: 40848808
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Oxygen Sensing in Osteocytes: From Physiology to Age-related Osteoporosis.Curr Osteoporos Rep. 2025 Jun 21;23(1):28. doi: 10.1007/s11914-025-00920-7. Curr Osteoporos Rep. 2025. PMID: 40542900 Review.
-
Analysis and Imaging of Osteocytes.J Vis Exp. 2024 Nov 29;(213):10.3791/64699. doi: 10.3791/64699. J Vis Exp. 2024. PMID: 39671327
References
-
- Anderson E, Kaliyamoorthy S, Iwan J, Alexander D, Knothe Tate M (2005) Nano? Microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes. Ann Biomed Eng 33:52–62 - PubMed
-
- Burger E (1993) Influence of mechanical factors on bone formation, resorption and growth in vitro. Bone 7:37–56
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

