Patient-derived enteroids provide a platform for the development of therapeutic approaches in microvillus inclusion disease
- PMID: 37643022
- PMCID: PMC10575727
- DOI: 10.1172/JCI169234
Patient-derived enteroids provide a platform for the development of therapeutic approaches in microvillus inclusion disease
Abstract
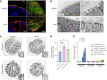
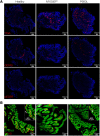
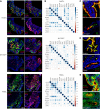
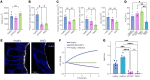
Microvillus inclusion disease (MVID), caused by loss-of-function mutations in the motor protein myosin Vb (MYO5B), is a severe infantile disease characterized by diarrhea, malabsorption, and acid/base instability, requiring intensive parenteral support for nutritional and fluid management. Human patient-derived enteroids represent a model for investigation of monogenic epithelial disorders but are a rare resource from MVID patients. We developed human enteroids with different loss-of function MYO5B variants and showed that they recapitulated the structural changes found in native MVID enterocytes. Multiplex immunofluorescence imaging of patient duodenal tissues revealed patient-specific changes in localization of brush border transporters. Functional analysis of electrolyte transport revealed profound loss of Na+/H+ exchange (NHE) activity in MVID patient enteroids with near-normal chloride secretion. The chloride channel-blocking antidiarrheal drug crofelemer dose-dependently inhibited agonist-mediated fluid secretion. MVID enteroids exhibited altered differentiation and maturation versus healthy enteroids. γ-Secretase inhibition with DAPT recovered apical brush border structure and functional Na+/H+ exchange activity in MVID enteroids. Transcriptomic analysis revealed potential pathways involved in the rescue of MVID cells including serum/glucocorticoid-regulated kinase 2 (SGK2) and NHE regulatory factor 3 (NHERF3). These results demonstrate the utility of patient-derived enteroids for developing therapeutic approaches to MVID.
Keywords: Drug therapy; Epithelial transport of ions and water; Gastroenterology; Genetic diseases.
Conflict of interest statement
Figures



Update of
-
Therapy Development for Microvillus Inclusion Disease using Patient-derived Enteroids.bioRxiv [Preprint]. 2023 Jan 29:2023.01.28.526036. doi: 10.1101/2023.01.28.526036. bioRxiv. 2023. Update in: J Clin Invest. 2023 Oct 16;133(20):e169234. doi: 10.1172/JCI169234. PMID: 36747680 Free PMC article. Updated. Preprint.

