Exploiting Mass Spectrometry to Unlock the Mechanism of Nanoparticle-Induced Inflammasome Activation
- PMID: 37643371
- PMCID: PMC10510732
- DOI: 10.1021/acsnano.3c05600
Exploiting Mass Spectrometry to Unlock the Mechanism of Nanoparticle-Induced Inflammasome Activation
Abstract
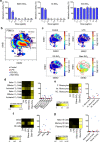
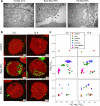
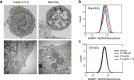
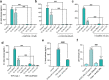
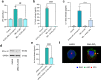
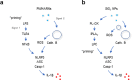
Nanoparticles (NPs) elicit sterile inflammation, but the underlying signaling pathways are poorly understood. Here, we report that human monocytes are particularly vulnerable to amorphous silica NPs, as evidenced by single-cell-based analysis of peripheral blood mononuclear cells using cytometry by time-of-flight (CyToF), while silane modification of the NPs mitigated their toxicity. Using human THP-1 cells as a model, we observed cellular internalization of silica NPs by nanoscale secondary ion mass spectrometry (nanoSIMS) and this was confirmed by transmission electron microscopy. Lipid droplet accumulation was also noted in the exposed cells. Furthermore, time-of-flight secondary ion mass spectrometry (ToF-SIMS) revealed specific changes in plasma membrane lipids, including phosphatidylcholine (PC) in silica NP-exposed cells, and subsequent studies suggested that lysophosphatidylcholine (LPC) acts as a cell autonomous signal for inflammasome activation in the absence of priming with a microbial ligand. Moreover, we found that silica NPs elicited NLRP3 inflammasome activation in monocytes, whereas cell death transpired through a non-apoptotic, lipid peroxidation-dependent mechanism. Together, these data further our understanding of the mechanism of sterile inflammation.
Keywords: cell death; inflammasome; mass spectrometry; monocyte; silica nanoparticles.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.P. is a former employee of Nouryon PPC, a company producing colloidal silica. Nouryon PPC had no input on the paper. The other authors declare no conflicts of interest.
Figures


References
-
- Cassel S. L.; Eisenbarth S. C.; Iyer S. S.; Sadler J. J.; Colegio O. R.; Tephly L. A.; Carter A. B.; Rothman P. B.; Flavell R. A.; Sutterwala F. S. The Nalp3 Inflammasome is Essential for the Development of Silicosis. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 9035–9040. 10.1073/pnas.0803933105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

