Phase II Trial of Dabrafenib Plus Trametinib in Relapsed/Refractory BRAF V600-Mutant Pediatric High-Grade Glioma
- PMID: 37643378
- PMCID: PMC10666989
- DOI: 10.1200/JCO.23.00558
Phase II Trial of Dabrafenib Plus Trametinib in Relapsed/Refractory BRAF V600-Mutant Pediatric High-Grade Glioma
Abstract
Purpose: BRAF V600 mutation is detected in 5%-10% of pediatric high-grade gliomas (pHGGs), and effective treatments are limited. In previous trials, dabrafenib as monotherapy or in combination with trametinib demonstrated activity in children and adults with relapsed/refractory BRAF V600-mutant HGG.
Methods: This phase II study evaluated dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600-mutant pHGG. The primary objective was overall response rate (ORR) by independent review by Response Assessment in Neuro-Oncology criteria. Secondary objectives included ORR by investigator determination, duration of response (DOR), progression-free survival, overall survival (OS), and safety.
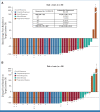
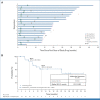
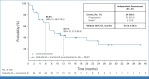
Results: A total of 41 pediatric patients with previously treated BRAF V600-mutant HGG were enrolled. At primary analysis, median follow-up was 25.1 months, and 51% of patients remained on treatment. Sixteen of 20 discontinuations were due to progressive disease in this relapsed/refractory pHGG population. Independently assessed ORR was 56% (95% CI, 40 to 72). Median DOR was 22.2 months (95% CI, 7.6 months to not reached [NR]). Fourteen deaths were reported. Median OS was 32.8 months (95% CI, 19.2 months to NR). The most common all-cause adverse events (AEs) were pyrexia (51%), headache (34%), and dry skin (32%). Two patients (5%) had AEs (both rash) leading to discontinuation.
Conclusion: In relapsed/refractory BRAF V600-mutant pHGG, dabrafenib plus trametinib improved ORR versus previous trials of chemotherapy in molecularly unselected patients with pHGG and was associated with durable responses and encouraging survival. These findings suggest that dabrafenib plus trametinib is a promising targeted therapy option for children and adolescents with relapsed/refractory BRAF V600-mutant HGG.
Trial registration: ClinicalTrials.gov NCT02684058.
Conflict of interest statement
Phase II Trial of Dabrafenib Plus Trametinib in Relapsed/Refractory
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Darren R. Hargrave
Keita Terashima
Junichi Hara
Santhosh A. Upadhyaya
Felix Sahm
Eric Bouffet
Roger J. Packer
Olaf Witt
Larissa Sandalic
Mark Russo
Kenneth J. Cohen
No other potential conflicts of interest were reported.
Figures
Comment in
-
Dabrafenib-trametinib is effective in paediatric high-grade glioma.Nat Rev Clin Oncol. 2023 Nov;20(11):734. doi: 10.1038/s41571-023-00820-8. Nat Rev Clin Oncol. 2023. PMID: 37726419 No abstract available.
References
-
- Bavle A, Chintagumpala M: Pediatric high-grade glioma: A review of biology, prognosis, and treatment. J Radiat Oncol 7:7-15, 2018
-
- Chatwin HV, Cruz Cruz J, Green AL: Pediatric high-grade glioma: Moving toward subtype-specific multimodal therapy. FEBS J 288:6127-6141, 2021 - PubMed
-
- Lashford LS, Thiesse P, Jouvet A, et al. : Temozolomide in malignant gliomas of childhood: A United Kingdom Children’s Cancer Study Group and French Society for Pediatric Oncology Intergroup study. J Clin Oncol 20:4684-4691, 2002 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

