Preclinical Study on Biodistribution of Mesenchymal Stem Cells after Local Transplantation into the Brain
- PMID: 37643762
- PMCID: PMC10686801
- DOI: 10.15283/ijsc23062
Preclinical Study on Biodistribution of Mesenchymal Stem Cells after Local Transplantation into the Brain
Abstract
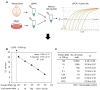
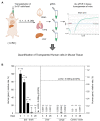
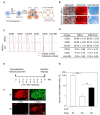
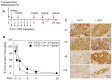
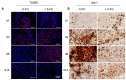
Therapeutic efficacy of mesenchymal stem cells (MSCs) is determined by biodistribution and engraftment in vivo. Compared to intravenous infusion, biodistribution of locally transplanted MSCs are partially understood. Here, we performed a pharmacokinetics (PK) study of MSCs after local transplantation. We grafted human MSCs into the brains of immune-compromised nude mice. Then we extracted genomic DNA from brains, lungs, and livers after transplantation over a month. Using quantitative polymerase chain reaction with human Alu-specific primers, we analyzed biodistribution of the transplanted cells. To evaluate the role of residual immune response in the brain, MSCs expressing a cytosine deaminase (MSCs/CD) were used to ablate resident immune cells at the injection site. The majority of the Alu signals mostly remained at the injection site and decreased over a week, finally becoming undetectable after one month. Negligible signals were transiently detected in the lung and liver during the first week. Suppression of Iba1-positive microglia in the vicinity of the injection site using MSCs/CD prolonged the presence of the Alu signals. After local transplantation in xenograft animal models, human MSCs remain predominantly near the injection site for limited time without disseminating to other organs. Transplantation of human MSCs can locally elicit an immune response in immune compromised animals, and suppressing resident immune cells can prolong the presence of transplanted cells. Our study provides valuable insights into the in vivo fate of locally transplanted stem cells and a local delivery is effective to achieve desired dosages for neurological diseases.
Keywords: Brain; Immune response; Mesenchymal stem cell; Pharmacokinetics; Real-time polymerase chain reaction; Transplantation.
Conflict of interest statement
NB, JHJ, DYC, and HSK are employees of and stock and/or option holders in CELLeBRAIN, Ltd.
Figures
References
LinkOut - more resources
Full Text Sources

