Neonatal and maternal outcomes following SARS-CoV-2 infection and COVID-19 vaccination: a population-based matched cohort study
- PMID: 37644002
- PMCID: PMC10465539
- DOI: 10.1038/s41467-023-40965-9
Neonatal and maternal outcomes following SARS-CoV-2 infection and COVID-19 vaccination: a population-based matched cohort study
Abstract
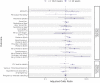
Understanding the impact of SARS-CoV-2 infection and COVID-19 vaccination in pregnancy on neonatal and maternal outcomes informs clinical decision-making. Here we report a national, population-based, matched cohort study to investigate associations between SARS-CoV-2 infection and, separately, COVID-19 vaccination just before or during pregnancy and the risk of adverse neonatal and maternal outcomes among women in Scotland with a singleton pregnancy ending at ≥20 weeks gestation. Neonatal outcomes are stillbirth, neonatal death, extended perinatal mortality, preterm birth (overall, spontaneous, and provider-initiated), small-for-gestational age, and low Apgar score. Maternal outcomes are admission to critical care or death, venous thromboembolism, hypertensive disorders of pregnancy, and pregnancy-related bleeding. We use conditional logistic regression to derive odds ratios adjusted for socio-demographic and clinical characteristics (aORs). We find that infection is associated with an increased risk of preterm (aOR=1.36, 95% Confidence Interval [CI] = 1.16-1.59) and very preterm birth (aOR = 1.90, 95% CI 1.20-3.02), maternal admission to critical care or death (aOR=1.72, 95% CI = 1.39-2.12), and venous thromboembolism (aOR = 2.53, 95% CI = 1.47-4.35). We find no evidence of increased risk for any of our outcomes following vaccination. These data suggest SARS-CoV-2 infection during pregnancy is associated with adverse neonatal and maternal outcomes, and COVID-19 vaccination remains a safe way for pregnant women to protect themselves and their babies against infection.
© 2023. Springer Nature Limited.
Conflict of interest statement
A.S. and C.R. were members of the Scottish Government’s COVID-19 Advisory Group. A.S. is a member of the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) risk stratification subgroup and the Scottish Government’s Committee on Pandemic Preparedness. C.R. is a member of the Scientific Pandemic Influenza Group on Modelling and the MHRA Covid 19 Vaccine Benefit and Risk Working Group. AS is a member of AstraZeneca’s Thrombotic Thrombocytopenic Advisory Group. All roles are unremunerated. S.V.K. was co-chair of Scottish Government’s Expert Reference Group on Ethnicity and COVID-19. All other authors declare no competing interests.
Figures
References
-
- NHS England. Saving Babies’ Lives Version Two (NHS England, 2019).
-
- Royal College of Midwives, Royal College of Obstetricians & Gyneacologists. Coronavirus (COVID-19) Infection in Pregnancy. Information for healthcare professionals (2022). https://www.rcog.org.uk/media/xsubnsma/2022-03-07-coronavirus-covid-19-i....
-
- National Institutes of Health. COVID-19 Treatments Guidelines - Special Considerations in Prengnacy (2022). https://www.covid19treatmentguidelines.nih.gov/special-populations/pregn...].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

