Bcl-2 inhibition combined with PPARα activation synergistically targets leukemic stem cell-like cells in acute myeloid leukemia
- PMID: 37644011
- PMCID: PMC10465498
- DOI: 10.1038/s41419-023-06075-6
Bcl-2 inhibition combined with PPARα activation synergistically targets leukemic stem cell-like cells in acute myeloid leukemia
Abstract
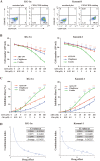
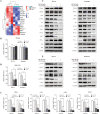
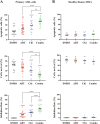
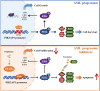
Persistence of leukemic stem cells (LSCs) is one of the determining factors to acute myeloid leukemia (AML) treatment failure and responsible for the poor prognosis of the disease. Hence, novel therapeutic strategies that target LSCs are crucial for treatment success. We investigated if targeting Bcl-2 and peroxisome proliferator activated receptor α (PPARα), two distinct cell survival regulating mechanisms could eliminate LSCs. This study demonstrate that the Bcl-2 inhibitor venetoclax combined with the PPARα agonist chiglitazar resulted in synergistic killing of LSC-like cell lines and CD34+ primary AML cells while sparing their normal counterparts. Furthermore, the combination regimen significantly suppressed AML progression in patient-derived xenograft (PDX) mouse models. Mechanistically, chiglitazar-mediated PPARα activation inhibited the transcriptional activity of the PIK3AP1 gene promoter and down-regulated the PI3K/Akt signaling pathway and anti-apoptotic Bcl-2 proteins, leading to cell proliferation inhibition and apoptosis induction, which was synergized with venetoclax. These findings suggest that combinatorial Bcl-2 inhibition and PPARα activation selectively eliminates AML cells in vivo and vitro, representing an effective therapy for patients with relapsed and refractory AML.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Combinations of HDAC Inhibitor and PPAR Agonist Induce Ferroptosis of Leukemic Stem Cell-like Cells in Acute Myeloid Leukemia.Clin Cancer Res. 2024 Dec 2;30(23):5430-5444. doi: 10.1158/1078-0432.CCR-24-0796. Clin Cancer Res. 2024. PMID: 39321217
-
Therapeutic inhibition of PPARα-HIF1α-PGK1 signaling targets leukemia stem and progenitor cells in acute myeloid leukemia.Cancer Lett. 2023 Feb 1;554:215997. doi: 10.1016/j.canlet.2022.215997. Epub 2022 Nov 15. Cancer Lett. 2023. PMID: 36396101
-
The pan-Bcl2 Inhibitor AT101 Activates the Intrinsic Apoptotic Pathway and Causes DNA Damage in Acute Myeloid Leukemia Stem-Like Cells.Target Oncol. 2017 Oct;12(5):677-687. doi: 10.1007/s11523-017-0509-2. Target Oncol. 2017. PMID: 28710745
-
Shutting Down Acute Myeloid Leukemia and Myelodysplastic Syndrome with BCL-2 Family Protein Inhibition.Curr Hematol Malig Rep. 2018 Aug;13(4):256-264. doi: 10.1007/s11899-018-0464-8. Curr Hematol Malig Rep. 2018. PMID: 29982865 Review.
-
Cell signaling pathways as molecular targets to eliminate AML stem cells.Crit Rev Oncol Hematol. 2021 Apr;160:103277. doi: 10.1016/j.critrevonc.2021.103277. Epub 2021 Mar 11. Crit Rev Oncol Hematol. 2021. PMID: 33716201 Review.
Cited by
-
Advances in the application of patient-derived xenograft models in acute leukemia resistance.Cancer Drug Resist. 2025 May 28;8:23. doi: 10.20517/cdr.2025.18. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40510031 Free PMC article. Review.
-
Investigating resistance to 5-Azacytidine and Venetoclax in PDX models of MDS/AML.Front Oncol. 2025 Jan 7;14:1414950. doi: 10.3389/fonc.2024.1414950. eCollection 2024. Front Oncol. 2025. PMID: 39839764 Free PMC article.
-
Leukemic Stem Cells and Hematological Malignancies.Int J Mol Sci. 2024 Jun 17;25(12):6639. doi: 10.3390/ijms25126639. Int J Mol Sci. 2024. PMID: 38928344 Free PMC article. Review.
-
Stemness regulation in prostate cancer: prostate cancer stem cells and targeted therapy.Ann Med. 2025 Dec;57(1):2442067. doi: 10.1080/07853890.2024.2442067. Epub 2024 Dec 23. Ann Med. 2025. PMID: 39711287 Free PMC article. Review.
-
The role of BUD31 in clear cell renal cell carcinoma: prognostic significance, alternative splicing, and tumor immune environment.Clin Exp Med. 2024 Aug 13;24(1):191. doi: 10.1007/s10238-024-01451-8. Clin Exp Med. 2024. PMID: 39136845 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

