Harnessing metabolism of hepatic macrophages to aid liver regeneration
- PMID: 37644019
- PMCID: PMC10465526
- DOI: 10.1038/s41419-023-06066-7
Harnessing metabolism of hepatic macrophages to aid liver regeneration
Abstract
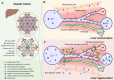
Liver regeneration is a dynamic and regulated process that involves inflammation, granulation, and tissue remodeling. Hepatic macrophages, abundantly distributed in the liver, are essential components that actively participate in each step to orchestrate liver regeneration. In the homeostatic liver, resident macrophages (Kupffer cells) acquire a tolerogenic phenotype and contribute to immunological tolerance. Following toxicity-induced damage or physical resection, Kupffer cells as well as monocyte-derived macrophages can be activated and promote an inflammatory process that supports the survival and activation of hepatic myofibroblasts and thus promotes scar tissue formation. Subsequently, these macrophages, in turn, exhibit the anti-inflammatory effects critical to extracellular matrix remodeling during the resolution stage. However, continuous damage-induced chronic inflammation generally leads to hepatic macrophage dysfunction, which exacerbates hepatocellular injury and triggers further liver fibrosis and even cirrhosis. Emerging macrophage-targeting strategies have shown efficacy in both preclinical and clinical studies. Increasing evidence indicates that metabolic rewiring provides substrates for epigenetic modification, which endows monocytes/macrophages with prolonged "innate immune memory". Therefore, it is reasonable to conceive novel therapeutic strategies for metabolically reprogramming macrophages and thus mediate a homeostatic or reparative process for hepatic inflammation management and liver regeneration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

