Ocular steroidome in human eyes and in eyes with complex central serous chorioretinopathy (CSCR)
- PMID: 37644063
- PMCID: PMC10465571
- DOI: 10.1038/s41598-023-41126-0
Ocular steroidome in human eyes and in eyes with complex central serous chorioretinopathy (CSCR)
Abstract
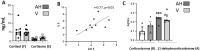
The exact link between systemic and ocular endogenous corticoids (steroidome) is unclear and whether the ocular steroidome is altered in CSCR eyes is unknown. The aims of this study were to analyze the human steroidome in the aqueous humor as a function of age, sex and time of the day, to correlate systemic and ocular steroidome and to analyze the ocular steroidome in long lasting complex inactive CSCR. Based on our results, we present two CSCR cases treated by the combination of oral mineralocorticoid antagonist and glucocorticoids drops. In a cross-sectional study, aqueous humor (AH) was collected between 8am and 6 pm from 50 unaffected individuals (25 men and 25 women) and from 14 patients with chronic CSCR, during cataract surgery. In addition, simultaneous serum and AH were collected from 27 individuals undergoing cataract surgery and, simultaneous AH and vitreous were collected from 9 patients undergoing cataract and vitrectomy to estimate corticoids levels in the different compartments. The steroidome was determined using a LC-MS/MS method that quantifies 13 endogenous corticoids from the gluco, mineralocorticoid and androgen pathways. In AH and vitreous, the highest corticoid level is reached by cortisol (F), that represents less than 10% of F serum level. The cortisol levels in the serum did not correlate with ocular cortisol levels. Serum and ocular cortisone (E) levels correlate, although less than 5% of circulating E reaches the eye. The only mineralocorticoids measured in the AH were corticosterone (B) and its inactive form, the 11-desoxycorticosterone (A). There was no influence of circadian rhythm on cortisol ocular levels and there was no correlation between the age or the sex and the level of F, E, A, and B. In eyes with chronic inactive CSCR, the levels of the active glucocorticoid form F was lower than in control eyes and the F/E ratio was reduced by 50% but the B/A ratio was higher indicating imbalance towards active mineralocorticoids. Base on this observation, we propose to combine an antagonist of the mineralocorticoid receptor together with topical glucocorticoids in two CSCR patients, resistant to all other treatments, with favorable outcome. Our results indicate that the ocular psteroidome is highly regulated suggesting a local metabolism of ocular corticoids. In eyes with long-lasting complex inactive CSCR, the steroidome analysis shows lower active glucocorticoids and higher active mineralocorticoids.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Daruich A, et al. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015;48:82–118. - PubMed
-
- Mrejen S, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–588. - PubMed
-
- Chhablani J, Cohen FB, Central Serous Chorioretinopathy International Group Multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol. Retina. 2020;4:1043–1046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

