2-Photon imaging of fluorescent proteins in living swine
- PMID: 37644074
- PMCID: PMC10465491
- DOI: 10.1038/s41598-023-40638-z
2-Photon imaging of fluorescent proteins in living swine
Abstract
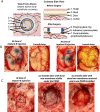
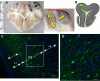
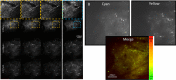
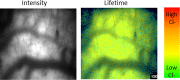
A common point of failure in translation of preclinical neurological research to successful clinical trials comes in the giant leap from rodent models to humans. Non-human primates are phylogenetically close to humans, but cost and ethical considerations prohibit their widespread usage in preclinical trials. Swine have large, gyrencencephalic brains, which are biofidelic to human brains. Their classification as livestock makes them a readily accessible model organism. However, their size has precluded experiments involving intravital imaging with cellular resolution. Here, we present a suite of techniques and tools for in vivo imaging of porcine brains with subcellular resolution. Specifically, we describe surgical techniques for implanting a synthetic, flexible, transparent dural window for chronic optical access to the neocortex. We detail optimized parameters and methods for injecting adeno-associated virus vectors through the cranial imaging window to express fluorescent proteins. We introduce a large-animal 2-photon microscope that was constructed with off-the shelf components, has a gantry design capable of accommodating animals > 80 kg, and is equipped with a high-speed digitizer for digital fluorescence lifetime imaging. Finally, we delineate strategies developed to mitigate the substantial motion artifact that complicates high resolution imaging in large animals, including heartbeat-triggered high-speed image stack acquisition. The effectiveness of this approach is demonstrated in sample images acquired from pigs transduced with the chloride-sensitive fluorescent protein SuperClomeleon.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
2-Photon imaging of fluorescent proteins in living swine.bioRxiv [Preprint]. 2023 Feb 14:2023.02.14.528533. doi: 10.1101/2023.02.14.528533. bioRxiv. 2023. Update in: Sci Rep. 2023 Aug 29;13(1):14158. doi: 10.1038/s41598-023-40638-z. PMID: 36824934 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

