Antibody response in elderly vaccinated four times with an mRNA anti-COVID-19 vaccine
- PMID: 37644113
- PMCID: PMC10465611
- DOI: 10.1038/s41598-023-41399-5
Antibody response in elderly vaccinated four times with an mRNA anti-COVID-19 vaccine
Abstract
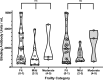
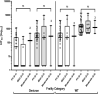
The humoral response after the fourth dose of a mRNA vaccine against COVID-19 has not been adequately described in elderly recipients, particularly those not exposed previously to SARS-CoV-2. Serum anti-RBD IgG levels (Abbott SARS-CoV-2 IgG II Quant assay) and neutralizing capacities (spike SARS-CoV-2 pseudovirus Wuhan and Omicron BA.1 variant) were measured after the third and fourth doses of a COVID-19 mRNA vaccine among 46 elderly residents (median age 85 years [IQR 81; 89]) of an assisted living facility. Among participants never infected by SARS-CoV-2, the mean serum IgG levels against RBD (2025 BAU/ml), 99 days after the fourth vaccine, was as high as 76 days after the third vaccine (1987 BAU/ml), and significantly higher (p = 0.030) when the latter were corrected for elapsed time. Neutralizing antibody levels against the historical Wuhan strain were significantly higher (Mean 1046 vs 1573; p = 0.002) and broader (against Omicron) (Mean 170 vs 375; p = 0.018), following the fourth vaccine. The six individuals with an Omicron breakthrough infection mounted strong immune responses for anti-RBD and neutralizing antibodies against the Omicron variant indicating that the fourth vaccine dose did not prevent a specific adaptation of the immune response. These findings point out the value of continued vaccine boosting in the elderly population.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Nordström P, Ballin M, Nordström A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: A nationwide, retrospective cohort study in Sweden. Lancet Reg. Health Eur. 2022;21:100466. doi: 10.1016/j.lanepe.2022.100466. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

