Animal models of heart failure with preserved ejection fraction (HFpEF): from metabolic pathobiology to drug discovery
- PMID: 37644131
- PMCID: PMC10770177
- DOI: 10.1038/s41401-023-01152-0
Animal models of heart failure with preserved ejection fraction (HFpEF): from metabolic pathobiology to drug discovery
Abstract
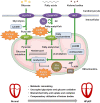
Heart failure (HF) with preserved ejection fraction (HFpEF) is currently a preeminent challenge for cardiovascular medicine. It has a poor prognosis, increasing mortality, and is escalating in prevalence worldwide. Despite accounting for over 50% of all HF patients, the mechanistic underpinnings driving HFpEF are poorly understood, thus impeding the discovery and development of mechanism-based therapies. HFpEF is a disease syndrome driven by diverse comorbidities, including hypertension, diabetes and obesity, pulmonary hypertension, aging, and atrial fibrillation. There is a lack of high-fidelity animal models that faithfully recapitulate the HFpEF phenotype, owing primarily to the disease heterogeneity, which has hampered our understanding of the complex pathophysiology of HFpEF. This review provides an updated overview of the currently available animal models of HFpEF and discusses their characteristics from the perspective of energy metabolism. Interventional strategies for efficiently utilizing energy substrates in preclinical HFpEF models are also discussed.
Keywords: HFpEF; animal models; diabetes; hypertension; metabolic inflexibility; obesity.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. doi: 10.1002/ejhf.2333. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

