Mammalian Nudt15 hydrolytic and binding activity on methylated guanosine mononucleotides
- PMID: 37644211
- PMCID: PMC10618335
- DOI: 10.1007/s00249-023-01678-5
Mammalian Nudt15 hydrolytic and binding activity on methylated guanosine mononucleotides
Abstract
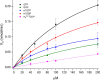
The Nudt15 enzyme of the NUDIX protein family is the subject of extensive study due to its action on thiopurine drugs used in the treatment of cancer and inflammatory diseases. In addition to thiopurines, Nudt15 is enzymatically active in vitro on several nucleotide substrates. It has also been suggested that this enzyme may play a role in 5'RNA turnover by hydrolyzing m7GDP, a product of mRNA decapping. However, no detailed studies on this substrate with Nudt15 are available. Here, we analyzed the enzymatic activity of Nudt15 with m7GDP, its triphosphate form m7GTP, and the trimethylated counterparts (m32,2,7GDP and m32,2,7GTP). Kinetic data revealed a moderate activity of Nudt15 toward these methylated mononucleotides compared to the dGTP substrate. However m7GDP and m32,2,7GDP showed a distinct stabilization of Nudt15 upon ligand binding, in the same range as dGTP, and thus these two mononucleotides may be used as leading structures in the design of small molecule binders of Nudt15.
Keywords: Differential scanning fluorimetry; Enzyme kinetics; Methylated mononucleotides; NUDIX family; Nudt15.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Carreras-Puigvert J, Zitnik M, Jemth AS, Carter M, Unterlass JE, Hallström B, Loseva O, Karem Z, Calderón-Montaño JM, Lindskog C, Edqvist PH, Matuszewski DJ, Ait Blal H, Berntsson RPA, Häggblad M, Martens U, Studham M, Lundgren B, Wählby C, Sonnhammer ELL, Lundberg E, Stenmark P, Zupan B, Helleday T. A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nat Commun. 2017;8(1):1541. doi: 10.1038/s41467-017-01642-w. - DOI - PMC - PubMed
-
- Carter M, Jemth AS, Hagenkort A, Page BD, Gustafsson R, Griese JJ, Gad H, Valerie NC, Desroses M, Boström J, Warpman Berglund U, Helleday T, Stenmark P. Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nat Commun. 2015;6:7871. doi: 10.1038/ncomms8871. - DOI - PMC - PubMed
-
- Gad H, Koolmeister T, Jemth AS, Eshtad S, Jacques SA, Ström CE, Svensson LM, Schultz N, Lundbäck T, Einarsdottir BO, Saleh A, Göktürk C, Baranczewski P, Svensson R, Berntsson RP, Gustafsson R, Strömberg K, Sanjiv K, Jacques-Cordonnier MC, Desroses M, Helleday T. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature. 2014;508(7495):215–221. doi: 10.1038/nature13181. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

