Block Copolymer-Templated, Single-Step Synthesis of Transition Metal Oxide Nanostructures for Sensing Applications
- PMID: 37644616
- PMCID: PMC10739603
- DOI: 10.1021/acsami.3c10439
Block Copolymer-Templated, Single-Step Synthesis of Transition Metal Oxide Nanostructures for Sensing Applications
Abstract
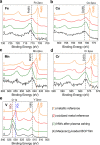
The synthesis of transition metal oxide nanostructures, thanks to their high surface-to-volume ratio and the resulting large fraction of surface atoms with high catalytic activity, is of prime importance for the development of new sensors and catalytic materials. Here, we report an economical, time-efficient, and easily scalable method of fabricating nanowires composed of vanadium, chromium, manganese, iron, and cobalt oxides by employing simultaneous block copolymer (BCP) self-assembly and selective sequestration of metal-organic acetylacetonate complexes within one of the BCP blocks. We discuss the mechanism and the primary factors that are responsible for the sequestration and conformal replication of the BCP template by the inorganic material that is obtained after the polymer template is removed. X-ray photoelectron spectroscopy (XPS) and powder X-ray diffraction (PXRD) studies indicate that the metal oxidation state in the nanowires produced by plasma ashing the BCP template closely matches that of the precursor complex and that their structure is amorphous, thus requiring high-temperature annealing in order to sinter them into a crystalline form. Finally, we demonstrate how the developed nanowire array fabrication scheme can be used to rapidly pattern a multilayered iron oxide nanomesh, which we then used to construct a prototype volatile organic compound sensor.
Keywords: block copolymers; directed self-assembly; gas sensors; solvent evaporation annealing; transition metal oxide nanowires.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Mirzaei A.; Ansari H. R.; Shahbaz M.; Kim J.-Y.; Kim H. W.; Kim S. S. Metal Oxide Semiconductor Nanostructure Gas Sensors with Different Morphologies. Chemosensors 2022, 10 (7), 289. 10.3390/chemosensors10070289. - DOI
-
- Botiz I.; Darling S. B. Optoelectronics Using Block Copolymers. Mater. Today 2010, 13 (5), 42–51. 10.1016/S1369-7021(10)70083-3. - DOI
-
- Kim J. H.; Jin H. M.; Yang G. G.; Han K. H.; Yun T.; Shin J. Y.; Jeong S.; Kim S. O. Smart Nanostructured Materials Based on Self-Assembly of Block Copolymers. Adv. Funct. Mater. 2020, 30 (2), 1902049 10.1002/adfm.201902049. - DOI
-
- Chen Y.; Xiong S. Directed Self-Assembly of Block Copolymers for Sub-10 Nm Fabrication. Int. J. Extrem. Manuf. 2020, 2 (3), 032006 10.1088/2631-7990/aba3ae. - DOI
Publication types
LinkOut - more resources
Full Text Sources

