Treatment outcomes of secukinumab in adult patients with moderate-to-severe plaque psoriasis in China: A real-world multicenter retrospective study
- PMID: 37644777
- PMCID: PMC10582683
- DOI: 10.1111/cts.13583
Treatment outcomes of secukinumab in adult patients with moderate-to-severe plaque psoriasis in China: A real-world multicenter retrospective study
Abstract
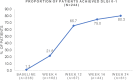
Secukinumab is effective in treating patients with moderate-to-severe plaque psoriasis. However, most studies assessing its effectiveness in routine clinical settings in China are mostly single-center studies with a limited sample size. The objective of this study was to assess secukinumab's efficacy, treatment patterns, and characteristics in patients with moderate-to-severe plaque psoriasis. This 24-week, multicenter (n = 5) retrospective study analyzed the data of Chinese adult patients with moderate-to-severe plaque psoriasis who initiated secukinumab treatment between May 2019 and March 2020. The Psoriasis Area and Severity Index (PASI), body surface area (BSA), Investigator's Global Assessment Modified 2011 (IGA mod 2011), and Dermatology Life Quality Index (DLQI) were assessed. Dermatologists documented the treatment dosage and modification reasons. Of the 244 secukinumab-naïve patients, most were men (73.4%, 179/244) and weighed 60-90 kg (72.8%, 177/243). The mean (SD) age at secukinumab initiation was 38.1 (11.6) years, and the disease duration was 13.5 (7.9) years. Most patients (97.1%, 237/244) received secukinumab 300 mg. At weeks 4, 12, 16, and 24, the proportion of patients achieving PASI 75 (≥75% reduction from baseline) was 40.0%, 92.1%, 88.4%, and 88.9%, respectively; PASI 90 was 15.0%, 73.7%, 81.4%, and 68.3%, respectively; and PASI 100 was 8.7%, 40.8%, 58.1%, and 41.3%, respectively. During the same periods, BSA and IGA mod 2011 showed similar improvement trends. An increasing proportion of patients achieved DLQI of 0-1 (21.6%, 65.7%, 75.0%, and 80.3%, respectively). Treatment modification was highest at week 12. The average interval between two administrations after week 4 was 62.95 days. Secukinumab was highly effective in improving the PASI, IGA, BSA, and DLQI in Chinese patients with moderate-to-severe plaque psoriasis throughout the first 24 weeks. The treatment pattern for Chinese patients differs from that in the clinical guidelines.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Yuling Shi, Chunlei Zhang, Chengzhi Lv, Bin Yang, Dingfeng Yang, Wei Li, Xin Guan, Na Liu, Ying Zhou, Gaojie Li, and Xiaohua Wang received support from Novartis in terms of funding, provision of study materials, operational and medical support, and medical writing. Zhidong Wang and Xiao Xiao are Novartis employees, who supported the investigators through funding, study materials, operational and medical support, and medical writing. All other authors declared no competing interests regarding this work.
Figures




References
-
- Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient‐membership survey. Arch Dermatol. 2001;137:280‐284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

