A phase 1 evaluation of the safety and tolerability of niraparib in combination with everolimus in advanced ovarian and breast cancers
- PMID: 37644890
- PMCID: PMC10557865
- DOI: 10.1002/cam4.6475
A phase 1 evaluation of the safety and tolerability of niraparib in combination with everolimus in advanced ovarian and breast cancers
Abstract
Objectives: Phase 1 trial to determine the safety and tolerability of everolimus and niraparib in patients with advanced ovarian and breast malignancies.
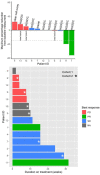
Results: Fourteen heavily pretreated patients were enrolled (12 high-grade serous ovarian cancer, 1 clear cell ovarian cancer, and 1 triple negative breast cancer). All patients were PARP naïve and received comprehensive genomic profiling prior to enrollment. Two DLTs were experienced in cohort 2 (niraparib 200 mg daily and everolimus 5 mg 3 days per week) with one patient experiencing prolonged thrombocytopenia and the other experiencing severe hypertension. Four additional patients were enrolled after dose de-escalation with one patient again experiencing severe hypertension leading to conclusion of the study. The most frequent grade 3 or greater adverse events were thrombocytopenia, hypertension, anemia, fatigue, neutropenia, and elevated alkaline phosphatase. Two patients had a PR and five patients had SD. ORR was 18% and the CBR was 45% in 11 evaluable patients. Median PFS was 6 months, and median OS is approximately 18 months with three patients still alive at the data cutoff.
Conclusions: The combination of everolimus and niraparib demonstrated significant toxicity at lower doses and is not feasible due to rapid onset and severe hypertension. This limitation possibly blunted the efficacy of the combination as PFS was modest, but OS was surprisingly robust due to three patients with ovarian cancer remaining alive with platinum refractory disease. Further investigation of multiagent blockade of the PI3K pathway combined with PARP is warranted.
Keywords: everolimus; niraparib; ovarian cancer; women's cancer.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial.Lancet Oncol. 2019 Oct;20(10):1409-1419. doi: 10.1016/S1470-2045(19)30515-7. Epub 2019 Aug 29. Lancet Oncol. 2019. PMID: 31474354 Clinical Trial.
-
A phase I study of the PARP inhibitor niraparib in combination with bevacizumab in platinum-sensitive epithelial ovarian cancer: NSGO AVANOVA1/ENGOT-OV24.Cancer Chemother Pharmacol. 2019 Oct;84(4):791-798. doi: 10.1007/s00280-019-03917-z. Epub 2019 Aug 2. Cancer Chemother Pharmacol. 2019. PMID: 31375879 Clinical Trial.
-
The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial.Lancet Oncol. 2013 Aug;14(9):882-92. doi: 10.1016/S1470-2045(13)70240-7. Epub 2013 Jun 28. Lancet Oncol. 2013. PMID: 23810788 Clinical Trial.
-
Homologous Recombination Deficiency Testing to Inform Patient Decisions About Niraparib Maintenance Therapy for High-Grade Serous or Endometrioid Epithelial Ovarian Cancer: A Health Technology Assessment.Ont Health Technol Assess Ser. 2023 Aug 10;23(5):1-188. eCollection 2023. Ont Health Technol Assess Ser. 2023. PMID: 37637244 Free PMC article.
-
The poly (ADP ribose) polymerase inhibitor niraparib: Management of toxicities.Gynecol Oncol. 2018 Apr;149(1):214-220. doi: 10.1016/j.ygyno.2018.01.011. Epub 2018 Feb 4. Gynecol Oncol. 2018. PMID: 29397193 Review.
Cited by
-
Case report: Response to everolimus in a patient with platinum resistant, high grade serous ovarian carcinoma with biallelic TSC2 inactivation.Front Oncol. 2024 Mar 27;14:1357980. doi: 10.3389/fonc.2024.1357980. eCollection 2024. Front Oncol. 2024. PMID: 38601768 Free PMC article.
-
Key Proteins of Replication Stress Response and Cell Cycle Control as Cancer Therapy Targets.Int J Mol Sci. 2024 Jan 19;25(2):1263. doi: 10.3390/ijms25021263. Int J Mol Sci. 2024. PMID: 38279263 Free PMC article. Review.
-
Oncogenic Pathways and Targeted Therapies in Ovarian Cancer.Biomolecules. 2024 May 15;14(5):585. doi: 10.3390/biom14050585. Biomolecules. 2024. PMID: 38785992 Free PMC article. Review.
References
-
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747‐752. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous