Chronic administration of a norepinephrine antagonist prevents and partially reverses escalation of cocaine self-administration
- PMID: 37644893
- PMCID: PMC10614571
- DOI: 10.1111/adb.13316
Chronic administration of a norepinephrine antagonist prevents and partially reverses escalation of cocaine self-administration
Abstract
Anxiety is a critical component of the development and maintenance of drug addiction; however, anti-anxiety medications such as benzodiazepines and beta-blockers (β-adrenergic receptor antagonists) are not used for the treatment of substance use disorder, except for the management of acute withdrawal syndrome. Preclinical studies have shown that beta-blockers may reduce stress-induced relapse; however, the effect of beta blockers on the escalation and maintenance of drug intake has not been tested. To address this issue, we chronically administered the β-adrenergic receptor antagonist propranolol during the escalation or maintenance of cocaine intake in a model of extended access (6 h) to cocaine self-administration (0.5 mg/kg). The behavioural specificity of propranolol was tested using a non-drug reward (saccharin). Daily administration of propranolol (15 mg/kg) prevented the development of escalation of cocaine self-administration and partially reversed self-administration after the establishment of escalation of intake. Moreover, propranolol dose-dependently decreased the motivation for cocaine tested under a progressive ratio schedule of reinforcement during the development of escalation and after maintenance. Finally, propranolol administration had no effect on the escalation and maintenance of saccharin self-administration. These results demonstrate that chronic treatment with propranolol provides therapeutic efficacy in reducing cocaine self-administration during the development and after the establishment of escalation of cocaine self-administration in an animal model relevant to cocaine use disorder. These results suggest that beta blockers should be further investigated as a target for medication development for the treatment of cocaine use disorder.
Keywords: beta-blocker; motivation; norepinephrine; stress; substance-use disorders; therapeutics.
© 2023 The Authors. Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Figures


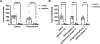
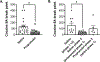

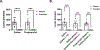
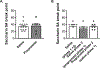
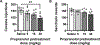
Similar articles
-
Escalation of intravenous cocaine self-administration, progressive-ratio performance, and reinstatement in rats selectively bred for high (HiS) and low (LoS) saccharin intake.Psychopharmacology (Berl). 2005 Feb;178(1):41-51. doi: 10.1007/s00213-004-1979-3. Epub 2004 Aug 27. Psychopharmacology (Berl). 2005. PMID: 15338102
-
Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake.Psychopharmacology (Berl). 2006 Jun;186(2):235-45. doi: 10.1007/s00213-006-0371-x. Epub 2006 Apr 5. Psychopharmacology (Berl). 2006. PMID: 16596398
-
The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access.Psychopharmacology (Berl). 2007 Sep;194(1):53-61. doi: 10.1007/s00213-007-0822-z. Epub 2007 May 27. Psychopharmacology (Berl). 2007. PMID: 17530475
-
Cocaine Seeking During Initial Abstinence Is Driven by Noradrenergic and Serotonergic Signaling in Hippocampus in a Sex-Dependent Manner.Neuropsychopharmacology. 2017 Jan;42(2):408-418. doi: 10.1038/npp.2016.150. Epub 2016 Aug 12. Neuropsychopharmacology. 2017. PMID: 27515792 Free PMC article.
-
Escalation of i.v. cocaine intake in peri-adolescent vs. adult rats selectively bred for high (HiS) vs. low (LoS) saccharin intake.Psychopharmacology (Berl). 2013 May;227(2):243-50. doi: 10.1007/s00213-012-2958-8. Epub 2013 Jan 10. Psychopharmacology (Berl). 2013. PMID: 23307070 Free PMC article.
Cited by
-
After a period of forced abstinence, rats treated with the norepinephrine neurotoxin DSP-4 still exhibit preserved food-seeking behavior and prefrontal cortex fos-expressing neurons.Heliyon. 2024 Jun 4;10(13):e32146. doi: 10.1016/j.heliyon.2024.e32146. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027623 Free PMC article.
-
Greater inhibition of female rat binge alcohol intake by adrenergic receptor blockers using a novel Two-Shot rat binge drinking model.Sci Rep. 2024 Jun 18;14(1):14029. doi: 10.1038/s41598-024-64565-9. Sci Rep. 2024. PMID: 38890353 Free PMC article.
-
Greater inhibition of female rat binge alcohol intake by adrenergic receptor blockers using a novel Two-Shot rat binge drinking model.Res Sq [Preprint]. 2024 May 29:rs.3.rs-4402198. doi: 10.21203/rs.3.rs-4402198/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Jun 18;14(1):14029. doi: 10.1038/s41598-024-64565-9. PMID: 38853968 Free PMC article. Updated. Preprint.
-
HIV-1 Tat and morphine interactions dynamically shift striatal monoamine levels and exploratory behaviors over time.J Neurochem. 2024 Mar;168(3):185-204. doi: 10.1111/jnc.16057. Epub 2024 Feb 3. J Neurochem. 2024. PMID: 38308495 Free PMC article.
-
Pharmacological Treatments for Methamphetamine Use Disorder: Current Status and Future Targets.Subst Abuse Rehabil. 2024 Aug 30;15:125-161. doi: 10.2147/SAR.S431273. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39228432 Free PMC article. Review.
References
-
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004. Aug;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. PMID: 15289279. - DOI - PubMed

