Underutilization of Systemic Therapy in Patients With NSCLC Undergoing Pneumonectomy: A Missed Opportunity for Survival
- PMID: 37644968
- PMCID: PMC10460993
- DOI: 10.1016/j.jtocrr.2023.100547
Underutilization of Systemic Therapy in Patients With NSCLC Undergoing Pneumonectomy: A Missed Opportunity for Survival
Abstract
Introduction: Recent trials have reported promising results with the addition of immunotherapy to chemotherapy for patients with locally advanced NSCLC, but in practice, the proportion of patients who receive systemic therapy (ST) has historically been low. Underutilization of ST may be particularly apparent in patients undergoing pneumonectomy, in whom the physiologic insult and surgical complications may preclude adjuvant therapy (ADJ). We, therefore, evaluated the use of ST for patients with NSCLC undergoing pneumonectomy.
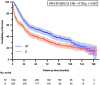
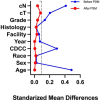
Methods: We queried the National Cancer Database, including all patients with NSCLC who underwent pneumonectomy between 2006 and 2018. Logistic regression was used to identify associations with ST and neo-ADJ (NEO). Overall survival was compared after propensity score matching (1:1) patients undergoing ST to those undergoing surgery alone using Kaplan-Meier and Cox regression methods.
Results: A total of 2619 patients were identified. Among these, 12% received NEO, 43% received ADJ, and 45% surgery alone. Age younger than 65 years (adjusted odds ratio [aOR] = 1.53, 95% confidence interval; [CI]: 1.10-2.11), Asian ethnicity (aOR = 2.68, 95% CI: 1.37-5.23), treatment at a high-volume center (aOR = 1.39, 95% CI: 1.06-1.81), and private insurance (aOR = 1.42, 95% CI: 1.05-1.94) were associated with NEO, whereas age younger than 65 years (aOR = 1.95, 95% CI: 1.61-2.38), comorbidity index less than or equal to 1 (aOR = 1.66, 95% CI: 1.29-2.16), and private insurance (aOR = 1.47, 95% CI: 1.20-1.80) were associated with any ST. In the matched cohort, ST was associated with better survival than surgery (adjusted hazard ratio = 0.67, 95% CI: 0.58-0.78).
Conclusions: A high proportion of patients who undergo pneumonectomy do not receive ST. Patient and socioeconomic factors are associated with the receipt of ST. Given its survival benefit, emphasis should be placed on multimodal treatment strategies, perhaps with greater consideration given to neoadjuvant approaches.
Keywords: Locally advanced tumor; Neoadjuvant therapy; Non–small cell lung cancer; Pneumonectomy; Systemic therapy.
© 2023 by the International Association for the Study of Lung Cancer.
Figures




Similar articles
-
Is underutilization of adjuvant therapy in resected non-small-cell lung cancer associated with socioeconomic disparities?Eur J Cardiothorac Surg. 2023 Dec 1;64(6):ezad383. doi: 10.1093/ejcts/ezad383. Eur J Cardiothorac Surg. 2023. PMID: 37952179 Free PMC article.
-
Surgery for Patients With cT3/4N2M0, Stage IIIB NSCLC. Is It Time to Redefine Resectability?JTO Clin Res Rep. 2024 Nov 14;6(1):100766. doi: 10.1016/j.jtocrr.2024.100766. eCollection 2025 Jan. JTO Clin Res Rep. 2024. PMID: 39758599 Free PMC article.
-
Induction chemoimmunotherapy with surgery versus concurrent chemoradiation followed by immunotherapy for stage III-N2 non-small cell lung cancer.J Thorac Cardiovasc Surg. 2024 Jun;167(6):1895-1905.e2. doi: 10.1016/j.jtcvs.2023.09.029. Epub 2023 Sep 16. J Thorac Cardiovasc Surg. 2024. PMID: 37722622
-
Comparison of Survival Rates After a Combination of Local Treatment and Systemic Therapy vs Systemic Therapy Alone for Treatment of Stage IV Non-Small Cell Lung Cancer.JAMA Netw Open. 2019 Aug 2;2(8):e199702. doi: 10.1001/jamanetworkopen.2019.9702. JAMA Netw Open. 2019. PMID: 31433481 Free PMC article.
-
Trends in Postoperative Intensity-Modulated Radiation Therapy Use and Its Association With Survival Among Patients With Incompletely Resected Non-Small Cell Lung Cancer.JAMA Netw Open. 2022 Sep 1;5(9):e2230704. doi: 10.1001/jamanetworkopen.2022.30704. JAMA Netw Open. 2022. PMID: 36074462 Free PMC article.
Cited by
-
Is underutilization of adjuvant therapy in resected non-small-cell lung cancer associated with socioeconomic disparities?Eur J Cardiothorac Surg. 2023 Dec 1;64(6):ezad383. doi: 10.1093/ejcts/ezad383. Eur J Cardiothorac Surg. 2023. PMID: 37952179 Free PMC article.
-
Sublobar resection is associated with less lymph nodes examined and lower delivery of adjuvant therapy in patients with 1.5- to 2.0-cm clinical IA2 non-small-cell lung cancer: a retrospective cohort study.Eur J Cardiothorac Surg. 2024 Jan 2;65(1):ezad431. doi: 10.1093/ejcts/ezad431. Eur J Cardiothorac Surg. 2024. PMID: 38147358 Free PMC article.
-
The impact of neoadjuvant therapy on postoperative outcomes following sleeve lobectomy for locally advanced non-small cell lung cancer: a call for future investigation.J Thorac Dis. 2024 Apr 30;16(4):2687-2689. doi: 10.21037/jtd-23-1982. Epub 2024 Apr 18. J Thorac Dis. 2024. PMID: 38738233 Free PMC article. No abstract available.
-
Real-World Clinical Characteristics, Treatment Patterns, and Clinical Outcomes in US Patients with Stage I-III Resected NSCLC Without Known EGFR Mutations: The RESECT Study.Drugs Real World Outcomes. 2025 Jun;12(2):175-188. doi: 10.1007/s40801-025-00487-w. Epub 2025 May 6. Drugs Real World Outcomes. 2025. PMID: 40329045 Free PMC article.
-
Minimally invasive surgery for clinical T4 non-small-cell lung cancer: national trends and outcomes.Eur J Cardiothorac Surg. 2024 Mar 1;65(3):ezae009. doi: 10.1093/ejcts/ezae009. Eur J Cardiothorac Surg. 2024. PMID: 38263602 Free PMC article.
References
-
- Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–3559. - PubMed
-
- Thai A.A., Solomon B.J., Sequist L.V., Gainor J.F., Heist R.S. Lung cancer. Lancet. 2021;398:535–554. - PubMed
-
- Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. - PubMed
LinkOut - more resources
Full Text Sources

