Fatal acute-on-chronic liver failure following camrelizumab for hepatocellular carcinoma with HBsAg seroclearance: a case report and literature review
- PMID: 37644988
- PMCID: PMC10461443
- DOI: 10.3389/fmed.2023.1231597
Fatal acute-on-chronic liver failure following camrelizumab for hepatocellular carcinoma with HBsAg seroclearance: a case report and literature review
Abstract
In the last few years, immune checkpoint inhibitors (ICIs) have become major therapeutic agents for the treatment of advanced hepatocellular carcinoma (HCC). However, immunotherapy can activate hepatitis B virus (HBV), and immune clearance may lead to liver failure and even life-threatening conditions. Here we report a case of HCC with HBV-related cirrhosis that caused severe liver injury and rapidly progressed to fatal acute-on-chronic liver failure (ACLF) after only once application of camrelizumab; the patient underwent serological conversion of hepatitis B surface antigen (HBsAg) with liver injury. The patient's condition progressed rapidly. We added corticosteroids and applied plasma dialysis, along with tenofovir alafenamide (TAF) to control HBV. However, the patient eventually died of liver failure. To our knowledge, there are few reports of HBsAg clearance due to ICIs accompanied by fatal acute-on-chronic liver failure shortly after ICIs initiation. These results suggest that ICIs can cause fatal liver injury in a short term; in patients with chronic HBV infection, ICIs use may promote serological conversion of HBsAg.
Keywords: clearance of HBsAg; hepatitis B; immune checkpoint inhibitors (ICIs); immune-mediated liver injury caused by checkpoint inhibitors (ILICI); liver failure.
Copyright © 2023 Li, Wang, Tang and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
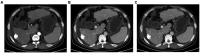
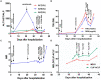
Figures

Similar articles
-
Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma.J Gastroenterol Hepatol. 2000 May;15 Suppl:E25-30. doi: 10.1046/j.1440-1746.2000.02097.x. J Gastroenterol Hepatol. 2000. PMID: 10921378
-
Impact of HBsAg seroclearance on late recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection.J Hepatol. 2022 Oct;77(4):939-946. doi: 10.1016/j.jhep.2022.05.014. Epub 2022 May 26. J Hepatol. 2022. PMID: 35643206
-
Risk of Hepatitis B Virus Reactivation in Patients Treated With Immunotherapy for Anti-cancer Treatment.Clin Gastroenterol Hepatol. 2022 Apr;20(4):898-907. doi: 10.1016/j.cgh.2021.06.019. Epub 2021 Jun 26. Clin Gastroenterol Hepatol. 2022. PMID: 34182151
-
Association Between Seroclearance of Hepatitis B Surface Antigen and Long-term Clinical Outcomes of Patients With Chronic Hepatitis B Virus Infection: Systematic Review and Meta-analysis.Clin Gastroenterol Hepatol. 2021 Mar;19(3):463-472. doi: 10.1016/j.cgh.2020.05.041. Epub 2020 May 27. Clin Gastroenterol Hepatol. 2021. PMID: 32473348
-
State of the art treatment of hepatitis B virus hepatocellular carcinoma and the role of hepatitis B surface antigen post-liver transplantation and resection.Liver Int. 2022 Feb;42(2):288-298. doi: 10.1111/liv.15124. Epub 2021 Dec 20. Liver Int. 2022. PMID: 34846790 Free PMC article. Review.
Cited by
-
Advances in mechanistic investigation and treatment of steroid-refractory ICI-induced liver injury.Clin Exp Med. 2025 Aug 11;25(1):288. doi: 10.1007/s10238-025-01721-z. Clin Exp Med. 2025. PMID: 40788551 Free PMC article. Review.
-
Management of Hepatitis B Virus and Hepatitis C Virus Infections in Patients with Cancer Receiving Immune Checkpoint Inhibitors.J Immunother Precis Oncol. 2024 May 2;7(2):111-121. doi: 10.36401/JIPO-23-28. eCollection 2024 May. J Immunother Precis Oncol. 2024. PMID: 38721408 Free PMC article. Review.
References
-
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. . Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

