This is a preprint.
Rapid model-guided design of organ-scale synthetic vasculature for biomanufacturing
- PMID: 37645046
- PMCID: PMC10462165
Rapid model-guided design of organ-scale synthetic vasculature for biomanufacturing
Update in
-
Rapid model-guided design of organ-scale synthetic vasculature for biomanufacturing.Science. 2025 Jun 12;388(6752):1198-1204. doi: 10.1126/science.adj6152. Epub 2025 Jun 12. Science. 2025. PMID: 40504910 Free PMC article.
Abstract
Our ability to produce human-scale bio-manufactured organs is critically limited by the need for vascularization and perfusion. For tissues of variable size and shape, including arbitrarily complex geometries, designing and printing vasculature capable of adequate perfusion has posed a major hurdle. Here, we introduce a model-driven design pipeline combining accelerated optimization methods for fast synthetic vascular tree generation and computational hemodynamics models. We demonstrate rapid generation, simulation, and 3D printing of synthetic vasculature in complex geometries, from small tissue constructs to organ scale networks. We introduce key algorithmic advances that all together accelerate synthetic vascular generation by more than 230 -fold compared to standard methods and enable their use in arbitrarily complex shapes through localized implicit functions. Furthermore, we provide techniques for joining vascular trees into watertight networks suitable for hemodynamic CFD and 3D fabrication. We demonstrate that organ-scale vascular network models can be generated in silico within minutes and can be used to perfuse engineered and anatomic models including a bioreactor, annulus, bi-ventricular heart, and gyrus. We further show that this flexible pipeline can be applied to two common modes of bioprinting with free-form reversible embedding of suspended hydrogels and writing into soft matter. Our synthetic vascular tree generation pipeline enables rapid, scalable vascular model generation and fluid analysis for bio-manufactured tissues necessary for future scale up and production.
Figures
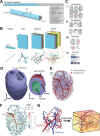



References
-
- Rouwkema J., Rivron N. C., Blitterswijk C. A., Trends in Biotechnology 26, 434 (2008). - PubMed
-
- Kakisis J., Liapis C. D., Breuer C., Sumpio B., Journal of Vascular Surgery 41, 349 (2005). - PubMed
-
- Conte M., FASEB Journal 12, 43 (1998). - PubMed
-
- Schlageter K. E., Molnar P., Lapin G. D., Groothuis D. R., Microvascular Research 58, 312 (1999). - PubMed
-
- Helmlinger G., Yuan F., Dellian M., Jain R. K., Nature Medicine 3, 177 (1997). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
