The Effect of Smoking on Inactivated and mRNA Vaccine Responses Applied to Prevent COVİD-19 in Multiple Sclerosis
- PMID: 37645088
- PMCID: PMC10461764
- DOI: 10.29399/npa.28503
The Effect of Smoking on Inactivated and mRNA Vaccine Responses Applied to Prevent COVİD-19 in Multiple Sclerosis
Abstract
Introduction: Coronavirus disease 2019 (COVID-19) is the biggest health challenge of recent times. Studies so far reveal that vaccination is the only way to prevent this pandemic. There may be factors that decrease or increase vaccine effectiveness. In multiple sclerosis (MS), some of these factors may cause changes in the effectiveness of the vaccine, depending on the nature of the disease and disease-modifying treatments (DMT). In this study, we aimed to investigate the relationship between antibody titer and smoking in non-treated and DMT-treated MS patients who received inactivated vaccine (Sinovac) and messenger RNA BNT162b2 (BioNTech) mRNA vaccines.
Method: Vaccine antibody responses were measured between 4-12 weeks after two doses of inactivated vaccine and mRNA vaccines. Patients were separated into 6 groups as: patients with MS without treatment PwMS w/o T, ocrelizumab, fingolimod, interferons (interferon beta-1a and interferon beta-1b), dimethyl fumarate, and teriflunomide. Antibody titers of smokers and non-smokers were compared for both vaccines and for each group.
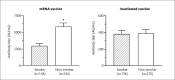
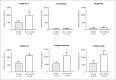
Results: The study included 798 patients. In the mRNA vaccine group, smokers (n=148; 2982±326 AU/mL) had lower antibody titers compared to the non-smokers (n=244; 5903±545 AU/mL) in total (p=0.020). In the inactivated vaccine group, no significant difference was detected between smokers (n=136; 383±51 AU/mL) and non-smokers (n=270; 388±49 AU/mL) in total (p=0.149). In both vaccine groups, patients receiving ocrelizumab and fingolimod had lower antibody titers than those receiving other DMTs or PwMS w/o T. In untreated MS patients, antibody levels in smokers were lower than in non-smokers in the mRNA vaccine group. No difference was found between antibody levels of smokers and non-smokers in any of the inactivated vaccine groups.
Conclusion: Ocrelizumab and fingolimod have lower antibody levels than PwMS w/o T or other DMTs in both mRNA and inactivated vaccine groups. Smoking decreases antibody levels in the mRNA vaccine group, while it has no effect in the inactivated vaccine group.
Keywords: COVID-19; disease modifying therapy; multiple sclerosis; smoking; vaccine.
Copyright: © 2023 Turkish Neuropsychiatric Society.
Conflict of interest statement
Conflict of Interest: S. Şen, M. Tütüncü, S. Demir, T. Gündüz, C. Uzunköprü, H. Gümüş have received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma. Aslı Tuncer has received honoraria or consultancy fees for partici- pating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma. Serkan Özakbaş has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck- Serono, Novartis, Teva, Biogen Idec/Gen Pharma. H. Efendi has received honoraria or consultancy fees for partici- pating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi Ibrahim Rana Karabudak has received honoraria for giving educational lec- tures, consultancy fees for participating advisory boards, and travel grants for attending scientific congresses or symposia from Roche, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey, Abdi Ibrahim Ilac, Deva and ARIS. Aksel Siva has received honoraria or consultancy fees for partici- pating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi Ibrahim Ilac. The rest of authors declare no conflict of interest with the study project.
Figures
Similar articles
-
mRNA versus inactivated virus COVID-19 vaccines in multiple sclerosis: Humoral responses and protectivity-Does it matter?Mult Scler Relat Disord. 2023 Jul;75:104761. doi: 10.1016/j.msard.2023.104761. Epub 2023 May 10. Mult Scler Relat Disord. 2023. PMID: 37247488 Free PMC article.
-
Humoral and Cellular Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with Multiple Sclerosis: An Israeli Multi-Center Experience Following 3 Vaccine Doses.Front Immunol. 2022 Apr 1;13:868915. doi: 10.3389/fimmu.2022.868915. eCollection 2022. Front Immunol. 2022. PMID: 35432335 Free PMC article.
-
COVID-19 Vaccine Response in People with Multiple Sclerosis Treated with Dimethyl Fumarate, Diroximel Fumarate, Natalizumab, Ocrelizumab, or Interferon Beta Therapy.Neurol Ther. 2023 Apr;12(2):687-700. doi: 10.1007/s40120-023-00448-x. Epub 2023 Feb 16. Neurol Ther. 2023. PMID: 36792812 Free PMC article.
-
Vaccine Response in Patients With Multiple Sclerosis Receiving Teriflunomide.Front Neurol. 2022 Feb 28;13:828616. doi: 10.3389/fneur.2022.828616. eCollection 2022. Front Neurol. 2022. PMID: 35295832 Free PMC article. Review.
-
Vaccine response in people with multiple sclerosis treated with fumarates.Mult Scler J Exp Transl Clin. 2023 Sep 6;9(3):20552173231191170. doi: 10.1177/20552173231191170. eCollection 2023 Jul-Sep. Mult Scler J Exp Transl Clin. 2023. PMID: 37692293 Free PMC article. Review.
Cited by
-
Factors Associated with Impaired Humoral Immune Response to mRNA Vaccines in Patients with Inflammatory Bowel Disease: A Matched-Cohort Analysis from the RisCoin Study.Vaccines (Basel). 2025 Jun 23;13(7):673. doi: 10.3390/vaccines13070673. Vaccines (Basel). 2025. PMID: 40733650 Free PMC article.
References
-
- Sormani MP, Schiavetti I, Landi D, Carmisciano L, De Rossi N, Cordioli C, et al. SARS-CoV-2 serology after COVID-19 in multiple sclerosis:an international cohort study. Mult Scler. 2022;28:1034–40. - PubMed
-
- Laroni A, Schiavetti I, Sormani MP, Uccelli A. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult Scler. 2021;27:2126–36. - PubMed
-
- World Health Organization (WHO) Coronavirus Disease (COVID-19) Dashboard. https: //covid19.who.int .
LinkOut - more resources
Full Text Sources