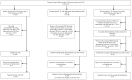
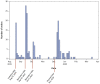
A pilot study of risk-stratified cervical cancer screening
- PMID: 37645164
- PMCID: PMC10445943
- DOI: 10.12688/openreseurope.13398.2
A pilot study of risk-stratified cervical cancer screening
Abstract
Background: Cervical screening programs target entire populations, although it is well established that cervical cancer risks can vary >100-fold based, in particular, on the woman's screening history. Since cervical screening switched to Human Papillomavirus (HPV) testing as the primary screening method, the risk differences are even larger as different HPV types may vary in associated cancer risk by 100 times. Furthermore, HPV infections with the most oncogenic types are declining dramatically because of HPV vaccination programs. Tailoring screening intensity based on the known cancer risk of the individual (risk-stratified screening) therefore has great potential to increase both the sensitivity and specificity. Within Horizon 2020 a major project for Risk-stratified Screening for Cervical Cancer (RISCC) has therefore been launched. We performed a pilot study of risk-stratified screening to evaluate feasibility and acceptability of offering vaginal HPV self-sampling tests to women with a higher risk of cervical cancer. Methods: We identified resident women who had had either i) atypical glandular cells in screening tests during the past six years (risk >150/100,000 woman-years) or ii) abnormal screening findings above the age of 50, but without sufficient follow-up (risk >65/100,000). The women were invited, either by short message service (SMS) or physical letters, to order an HPV self-sampling kit via the study web-platform. The returned self-collected samples were tested for HPV. If positive, women were invited for clinical follow-up. Results: Among 920 targeted women, 191 (21%) placed an order and 163 (18%) returned a self-collected sample. Among all tested samples, 19 (12%) were positive for hrHPV and 18 of these women attended clinical follow-up. Conclusions: SMS invitations to high-risk women complemented with physical letters are feasible and result in substantial requests for kits and submission of samples. Future work will focus on improving the efficiency of the procedure and further increasing attendance.
Keywords: Human Papillomavirus; SMS-based screening invitations; self-sampling.
Copyright: © 2022 Wang J et al.
Conflict of interest statement
Competing interests: JD has received grants to his institution for studies on HPV tests from Roche and Genomica, manufacturers of HPV tests. JW receives part of her salary from a research grant from Merck & Co. for project on HPV vaccine evaluation.
Figures
References
-
- World Health Organisation: To eliminate cervical cancer in the next 100 years, implementing an effective strategy is critical. (accessed 6 Dec 2020). Reference Source
-
- World Health Organisation: Launch of the Global Strategy to Accelerate the Elimination of Cervical Cancer. (accessed 6 Dec 2020). Reference Source
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous