Linking niche size and phylogenetic signals to predict future soil microbial relative abundances
- PMID: 37645222
- PMCID: PMC10461061
- DOI: 10.3389/fmicb.2023.1097909
Linking niche size and phylogenetic signals to predict future soil microbial relative abundances
Abstract
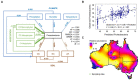
Bacteria provide ecosystem services (e.g., biogeochemical cycling) that regulate climate, purify water, and produce food and other commodities, yet their distribution and likely responses to change or intervention are difficult to predict. Using bacterial 16S rRNA gene surveys of 1,381 soil samples from the Biomes of Australian Soil Environment (BASE) dataset, we were able to model relative abundances of soil bacterial taxonomic groups and describe bacterial niche space and optima. Hold out sample validated hypothetical causal networks (structural equation models; SEM) were able to predict the relative abundances of bacterial taxa from environmental data and elucidate soil bacterial niche space. By using explanatory SEM properties as indicators of microbial traits, we successfully predicted soil bacterial response, and in turn potential ecosystem service response, to near-term expected changes in the Australian climate. The methods developed enable prediction of continental-scale changes in bacterial relative abundances, and demonstrate their utility in predicting changes in bacterial function and thereby ecosystem services. These capabilities will be strengthened in the future with growing genome-level data.
Keywords: environmental change; microbial traits; phylogenetic signal; soil; soil bacteria; structural equation model.
Copyright © 2023 Bissett, Mamet, Lamb and Siciliano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abouheif L. (1999). A method for testing the assumption of phylogenetic independence in comparative data. Evol. Ecol. Res. 1, 895–909.
-
- Angly F. E., Dennis P. G., Skarshewski A., Vanwonterghem I., Hugenholtz P., Tyson G. W. (2014). CopyRighter: a rapid tool for improving the accuracy of microbial community profiles through lineage-specific gene copy number correction. Microbiome 2:11. doi: 10.1186/2049-2618-2-11, PMID: - DOI - PMC - PubMed
-
- Anselin L. (1995). Local indicators of spatial association—LISA. Geogr. Anal. 27, 93–115. doi: 10.1111/j.1538-4632.1995.tb00338.x - DOI
LinkOut - more resources
Full Text Sources

