Assessment of the efficacy of SARS-CoV-2 vaccines in non-human primate studies: a systematic review
- PMID: 37645309
- PMCID: PMC10446071
- DOI: 10.12688/openreseurope.14375.1
Assessment of the efficacy of SARS-CoV-2 vaccines in non-human primate studies: a systematic review
Abstract
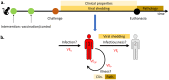
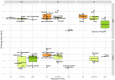
Background: The outbreak of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggered the rapid and successful development of vaccines to help mitigate the effect of COVID-19 and circulation of the virus. Vaccine efficacy is often defined as capacity of vaccines to prevent (severe) disease. However, the efficacy to prevent transmission or infectiousness is equally important at a population level. This is not routinely assessed in clinical trials. Preclinical vaccine trials provide a wealth of information about the presence and persistence of viruses in different anatomical sites. Methods: We systematically reviewed all available preclinical SARS-CoV-2 candidate vaccine studies where non-human primates were challenged after vaccination (PROSPERO registration: CRD42021231199). We extracted the underlying data, and recalculated the reduction in viral shedding. We summarized the efficacy of vaccines to reduce viral RNA shedding after challenge by standardizing and stratifying the results by different anatomical sites and diagnostic methods. We considered shedding of viral RNA as a proxy measure for infectiousness. Results: We found a marked heterogeneity between the studies in the experimental design and the assessment of the outcomes. The best performing vaccine candidate per study caused only low (6 out of 12 studies), or moderate (5 out of 12) reduction of viral genomic RNA, and low (5 out of 11 studies) or moderate (3 out of 11 studies) reduction of subgenomic RNA in the upper respiratory tract, as assessed with nasal samples. Conclusions: Since most of the tested vaccines only triggered a low or moderate reduction of viral RNA in the upper respiratory tract, we need to consider that most SARS-CoV-2 vaccines that protect against disease might not fully protect against infectiousness and vaccinated individuals might still contribute to SARS-CoV-2 transmission. Careful assessment of secondary attack rates from vaccinated individuals is warranted. Standardization in design and reporting of preclinical trials is necessary.
Keywords: Animal models; Preclinical data; SARS-CoV-2; Vaccine efficacy.
Copyright: © 2022 Counotte MJ et al.
Conflict of interest statement
No competing interests were disclosed.
Figures


References
-
- Reuters: Sinovac's coronavirus vaccine candidate approved for emergency use in China.2020. Reference Source
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
