Establishing the intracellular niche of obligate intracellular vacuolar pathogens
- PMID: 37645379
- PMCID: PMC10461009
- DOI: 10.3389/fcimb.2023.1206037
Establishing the intracellular niche of obligate intracellular vacuolar pathogens
Abstract
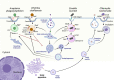
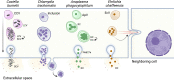
Obligate intracellular pathogens occupy one of two niches - free in the host cell cytoplasm or confined in a membrane-bound vacuole. Pathogens occupying membrane-bound vacuoles are sequestered from the innate immune system and have an extra layer of protection from antimicrobial drugs. However, this lifestyle presents several challenges. First, the bacteria must obtain membrane or membrane components to support vacuole expansion and provide space for the increasing bacteria numbers during the log phase of replication. Second, the vacuole microenvironment must be suitable for the unique metabolic needs of the pathogen. Third, as most obligate intracellular bacterial pathogens have undergone genomic reduction and are not capable of full metabolic independence, the bacteria must have mechanisms to obtain essential nutrients and resources from the host cell. Finally, because they are separated from the host cell by the vacuole membrane, the bacteria must possess mechanisms to manipulate the host cell, typically through a specialized secretion system which crosses the vacuole membrane. While there are common themes, each bacterial pathogen utilizes unique approach to establishing and maintaining their intracellular niches. In this review, we focus on the vacuole-bound intracellular niches of Anaplasma phagocytophilum, Ehrlichia chaffeensis, Chlamydia trachomatis, and Coxiella burnetii.
Keywords: Anaplasma; Chlamydia; Coxiella; Ehrlichia; egress; membrane; pathogen vacuole; vesicular trafficking.
Copyright © 2023 Clemente, Angara and Gilk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aeberhard L., Banhart S., Fischer M., Jehmlich N., Rose L., Koch S., et al. . (2015). The proteome of the isolated chlamydia trachomatis containing vacuole reveals a complex trafficking platform enriched for retromer components. PloS Pathog. 11, e1004883. doi: 10.1371/journal.ppat.1004883 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

