Neurological adverse events of mitotane in adrenocortical carcinoma: results of a pilot study
- PMID: 37645432
- PMCID: PMC10461107
- DOI: 10.3389/fonc.2023.1222002
Neurological adverse events of mitotane in adrenocortical carcinoma: results of a pilot study
Abstract
Introduction: Mitotane, the only drug approved by the Food and Drug Administration (FDA) for the treatment of adrenocortical carcinoma, is associated with several side effects including neurotoxicity. The aim of our study is to investigate the relationship between mitotane plasma levels and neurological toxicity.
Methods: We have considered five patients affected by adrenocortical carcinoma treated with mitotane. The neurological assessment included a neurological examination, an electroencephalogram, event-related potentials (P300), and a neuropsychological assessment. All of the patients were first considered at the onset of symptoms of neurotoxicity or when mitotanemia levels were above 18 mg/L, for the second time at mitotanemia normalization and subsequently at its further increase, or in case of persistent neurological abnormalities, some months after normalization.
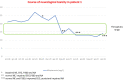
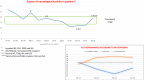
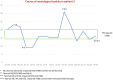
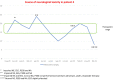
Results: At the first neurotoxicity, four patients showed impaired neurological examination, electroencephalogram, and P300; three patients had impaired neuropsychological assessment; one patient, only P300. At mitotanemia normalization, the neurological examination became normal in all patients and electroencephalogram normalized in one patient, improved in another one, continuing to be altered in the other three. P300 latency and neuropsychological assessment normalized in two patients and persisted altered in the patient experiencing long-term mitotane toxicity. At the third evaluation, in the patient with prolonged mitotane toxicity, the normal mitotanemia in the previous 9 months restored P300 and improved the electroencephalogram but not the neuropsychological assessment. In the two patients experiencing a further rise of mitotanemia, neurological examination was normal but P300 and electroencephalogram were altered.
Conclusion: The results of our study highlighted the presence of neurophysiological and neuropsychological abnormalities associated with mitotane values above 18 mg/L.
Keywords: P300; adrenocortical carcinoma; electroencephalogram; mitotane; neurological toxicity; neuropsychological testing.
Copyright © 2023 Mormando, Galiè, Bianchini, Lauretta, Puliani, Tanzilli, Anceschi, Simone, Petreri, Graziano, Pace and Appetecchia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hermsen IG, Fassnacht M, Terzolo M, Houterman S, den Hartigh J, Leboulleux S, et al. . Plasma concentrations of o,p'DDD, o,p'DDA, and o,p'DDE as predictors of tumor response to mitotane in adrenocortical carcinoma: results of a retrospective ENS@T multicenter study. J Clin Endocrinol Metab (2011) 96:1844–51. doi: 10.1210/jc.2010-2676 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

