A unique cytotoxic CD4+ T cell-signature defines critical COVID-19
- PMID: 37645435
- PMCID: PMC10461786
- DOI: 10.1002/cti2.1463
A unique cytotoxic CD4+ T cell-signature defines critical COVID-19
Abstract
Objectives: SARS-CoV-2 infection causes a spectrum of clinical disease presentation, ranging from asymptomatic to fatal. While neutralising antibody (NAb) responses correlate with protection against symptomatic and severe infection, the contribution of the T-cell response to disease resolution or progression is still unclear. As newly emerging variants of concern have the capacity to partially escape NAb responses, defining the contribution of individual T-cell subsets to disease outcome is imperative to inform the development of next-generation COVID-19 vaccines.
Methods: Immunophenotyping of T-cell responses in unvaccinated individuals was performed, representing the full spectrum of COVID-19 clinical presentation. Computational and manual analyses were used to identify T-cell populations associated with distinct disease states.
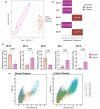
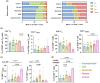
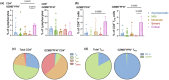
Results: Critical SARS-CoV-2 infection was characterised by an increase in activated and cytotoxic CD4+ lymphocytes (CTL). These CD4+ CTLs were largely absent in asymptomatic to severe disease states. In contrast, non-critical COVID-19 was associated with high frequencies of naïve T cells and lack of activation marker expression.
Conclusion: Highly activated and cytotoxic CD4+ T-cell responses may contribute to cell-mediated host tissue damage and progression of COVID-19. Induction of these potentially detrimental T-cell responses should be considered when developing and implementing effective COVID-19 control strategies.
Keywords: CD4‐CTLs; COVID‐19; SARS‐CoV‐2; T cells; spectral cytometry.
© 2023 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . WHO coronavirus (COVID‐19) dashboard. 2022. https://covid19.who.int
-
- Khoury DS, Cromer D, Reynaldi A et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med 2021; 27: 1205–1211. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
