Impact of clinical pharmacist-led intervention for drug-related problems in neonatal intensive care unit a randomized controlled trial
- PMID: 37645440
- PMCID: PMC10461390
- DOI: 10.3389/fphar.2023.1242779
Impact of clinical pharmacist-led intervention for drug-related problems in neonatal intensive care unit a randomized controlled trial
Abstract
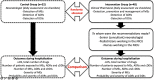
Introduction: Drug-related problems (DRPs) incidence is higher in neonatal intensive care units (NICUs), compared to other pediatric wards due to aspects like off-label medications, pharmacokinetic/dynamic variability, or organ dysfunction/immaturity. This study aimed to determine whether and to what extent a clinical pharmacist intervention improves medication safety and prevents DRPs [medication errors (MEs), adverse drug reactions (ADRs), drug-drug interactions (DDIs)]. Methods: A prospective, randomized, double blind, controlled study in NICU-admitted neonates was conducted. NICU patients were randomly assigned to the intervention (clinical pharmacist-led) (IG) or control group (standard care such as clinical diagnosis, pharmacotherapy) (CG). The clinical pharmacist was involved in the IG to identify-prevent-intervene MEs, or identify and monitor ADRs and DDIs. The primary outcome was the number of neonates who developed at least one DRP compared with those seen across IG and CG. Secondary outcomes included length of hospital stay, total number of drugs or DRP type. Results: Neonates were randomly assigned to CG (n = 52) or IG (n = 48). In total, 45%, 42%, and 16% of patients had at least 1 MEs, ADRs, and clinically significant DDIs, respectively. The number of patients with at least 1 ME was 28 (53%) and 17 (35%) in the CG and IG (p>0.05). The median (range) number of ME was higher in CG [1 (0-7)] than in IG [0 (0-4)] (p = 0.003). Applying regression analysis, the CG had 2.849 times more MEs than the IG (p<0.001). Furthermore, the number of patients (CG to IG) with at least one detected ADR or clinical DDI was 19 (36%) to 23 (47%) (p>0.05) and 4 (7%) to 12 (25%), respectively (p = 0.028). Conclusion: Clinical pharmacist availability to systematically and standardized identify, prevent and resolve DRPs among NICU patients is effective. Daily detailed clinical pharmacist observations and interventions enables prevention and monitoring of DRPs. Clinical Trial Registration ClinicalTrials.gov, identifier NCT04899960.
Keywords: adverse drug reactions; clinical pharmacist (CP); drug-drug interactions (DDIs); drug-related problems (DRPs); medication errors; pharmaceutical care; pharmacotherapy optimization.
Copyright © 2023 Yalçın, Kaşıkcı, Çelik, Allegaert, Demirkan and Yiğit.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Handling drug-related problems in rehabilitation patients: a randomized study.Int J Clin Pharm. 2012 Apr;34(2):382-8. doi: 10.1007/s11096-012-9623-5. Epub 2012 Mar 3. Int J Clin Pharm. 2012. PMID: 22388601 Clinical Trial.
-
Determine the impact of a structured pharmacist-led medication review - a controlled intervention study to optimise medication safety for residents in long-term care facilities.BMC Geriatr. 2022 Apr 9;22(1):307. doi: 10.1186/s12877-022-03025-3. BMC Geriatr. 2022. PMID: 35397527 Free PMC article. Clinical Trial.
-
Investigation of drug-related problems in patients hospitalized in chest disease wards: A randomized controlled trial.Front Pharmacol. 2023 Jan 10;13:1049289. doi: 10.3389/fphar.2022.1049289. eCollection 2022. Front Pharmacol. 2023. PMID: 36703759 Free PMC article.
-
The scope of drug-related problems in the home care setting.Int J Clin Pharm. 2018 Apr;40(2):325-334. doi: 10.1007/s11096-017-0581-9. Epub 2018 Jan 11. Int J Clin Pharm. 2018. PMID: 29322475 Review.
-
Evaluation of Pharmacist Medication Review Service in an Outpatient Heart Failure Clinic.J Pharm Pract. 2020 Dec;33(6):820-826. doi: 10.1177/0897190019842696. Epub 2019 May 5. J Pharm Pract. 2020. PMID: 31057060 Review.
Cited by
-
Improving Medication Safety Through Medication Reconciliation in Pediatric Neurology: Clinical Pharmacist Recommendations and Physician Uptake in a 13-Week Study.Children (Basel). 2025 May 12;12(5):625. doi: 10.3390/children12050625. Children (Basel). 2025. PMID: 40426804 Free PMC article.
-
Cost-benefit analysis of pharmacist early active consultation in patients with multidrug-resistant bacteria in China.Int J Clin Pharm. 2025 Jun;47(3):863-872. doi: 10.1007/s11096-025-01889-0. Epub 2025 Mar 20. Int J Clin Pharm. 2025. PMID: 40111584
-
Pharmaceutical interventions for drug-related problems in the neonatal intensive care unit: incidence, types, and acceptability.Front Pharmacol. 2024 May 30;15:1391657. doi: 10.3389/fphar.2024.1391657. eCollection 2024. Front Pharmacol. 2024. PMID: 38873432 Free PMC article.
References
-
- Burckart G. J. (2012). Clinical pharmacology and clinical pharmacy A marriage of necessity Figure 1. Eur. J. Hosp. Pharm. Sci. Pract. 19 (1), 19–21. 10.1136/ejhpharm-2011-000002 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous