Safety profiles of methylphenidate, amphetamine, and atomoxetine: analysis of spontaneous reports submitted to the food and drug administration adverse event reporting system
- PMID: 37645441
- PMCID: PMC10461182
- DOI: 10.3389/fphar.2023.1208456
Safety profiles of methylphenidate, amphetamine, and atomoxetine: analysis of spontaneous reports submitted to the food and drug administration adverse event reporting system
Abstract
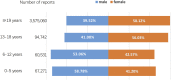
Background: Methylphenidate, atomoxetine, and Amphetamine are the three most commonly used medications approved by the United States Food and Drug Administration (FDA) for the treatment of attention deficit/hyperactivity disorder (ADHD). However, a comprehensive analysis of their safety profiles across various age groups and genders in real-world contexts has yet to be conducted. In this study, a pharmacovigilance analysis was performed using the FDA Adverse Event Reporting System (FAERS) database to examine differences in adverse events between methylphenidate, atomoxetine, and Amphetamine. Methods: From January 2014 to September 2022, FAERS reports listing "Methylphenidate," "Dexmethylphenidate," "Atomoxetine," "Amphetamine," "Lisdexamfetamine," "Dextroamphetamine," and "Methamphetamine" as primary suspects were analyzed after removing duplicate reports. We used the standardized Medical Dictionary for Regulatory Activities (MedDRA) query generalized search for adverse events at the preferred term level based on case reports. After filtering duplicate reports, disproportionality analysis was used to detect safety signals according to the proportional reporting ratio (PRR). In order to delve into potential safety concerns, we undertook a two-step analysis of the data. Initially, the data was segmented based on age cohorts: 0-5 years, 6-12 years, 13-18 years, and individuals aged ≥19 years. Following this, after partitioning the data into males and females within the 0-18 years age group, and similarly for those aged ≥19 years, further analysis was conducted. Results: The pharmacovigilance analysis uncovered substantial safety signals in the standardized MedDRA queries. Methylphenidate was associated with dyskinesia (PRR = 21.15), myocardial infarction (PRR = 12.32), and hypertension (PRR = 8.95) in children aged 0-5, 6-12, and 13-18 years, respectively, as well as neonatal exposures via breast milk (PRR = 14.10) in adults aged ≥19 years. Atomoxetine was linked to hostility/aggression (PRR = 15.77), taste and smell disorders (PRR = 6.75), and hostility/aggression (PRR = 6.74) in children aged 0-5, 6-12, and 13-18 years, respectively, as well as hostility/aggression (PRR = 14.00) in adults aged ≥19 years. Amphetamine was associated with psychosis and psychotic disorders (PRR = 16.78), hostility/aggression (PRR = 4.39), and Other ischaemic heart disease (PRR = 10.77) in children aged 0-5 years, 6-12 years, and 13-18 years, respectively, and hostility/aggression in adults aged ≥19 years (PRR = 9.16). Significant and noteworthy adverse event signals were also identified at the preferred term level. Specifically, methylphenidate was associated with myocardial infarction, acute myocardial infarction, coronary artery dissection, electrocardiogram QT prolonged, growth retardation, self-destructive behavior, suicidal ideation, and completed suicide. Atomoxetine was linked to electrocardiogram QT prolonged, growth retardation, and tic. Amphetamine was recorded for coronary artery dissection, suicidal ideation, and completed suicide. It was observed that male patients, including both children and adults, showed a more significant and frequent occurrence of adverse events compared to females, particularly in terms of cardiac disorders. The intensity and quantity of adverse event signals were distinctly different between the two genders, with males having a higher number of signals. All detected safety signals were confirmed using signals obtained from the disproportionality analysis. Conclusion: This pharmacovigilance analysis demonstrated significant variations in the safety profiles of methylphenidate, atomoxetine, and Amphetamine across different age groups and between different genders. Following an in-depth analysis of the FAERS database, we discerned prominent safety signals. Notably, the strength of the signals associated with coronary artery dissection induced by methylphenidate and amphetamine, as well as those related to suicide, demand particular attention. Consequently, it remains imperative to persist in monitoring these medications, assessing the associated risks, and carrying out comparative studies particularly geared towards ADHD drugs.
Keywords: FDA adverse events reporting system; amphetamine; atomoxetine; dexmethylphenidate; dextroamphetamine; lisdexamfetamine; methamphetamine; methylphenidate.
Copyright © 2023 Wei, Chen, Zhou, Liu, Zhang, Feng, Bai, Leng, Chang and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Anderson K. N., Ailes E. C., Danielson M., Lind J. N., Farr S. L., Broussard C. S., et al. (2018). Attention-deficit/hyperactivity disorder medication prescription claims among privately insured women aged 15-44 Years - United States, 2003-2015. MMWR Morb. Mortal. Wkly. Rep. 67 (2), 66–70. 10.15585/mmwr.mm6702a3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

