This is a preprint.
Elevated serotonin in mouse spinal dorsal horn is pronociceptive
- PMID: 37645759
- PMCID: PMC10461991
- DOI: 10.1101/2023.08.10.552838
Elevated serotonin in mouse spinal dorsal horn is pronociceptive
Update in
-
Elevated Serotonin in Mouse Spinal Dorsal Horn Is Pronociceptive.eNeuro. 2023 Dec 4;10(12):ENEURO.0293-23.2023. doi: 10.1523/ENEURO.0293-23.2023. Print 2023 Dec. eNeuro. 2023. PMID: 37945351 Free PMC article.
Abstract
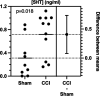
Serotonergic neurons in the rostral ventral medulla (RVM) contribute to bidirectional control of pain through modulation of spinal and trigeminal nociceptive networks. Deficits in this pathway are believed to contribute to pathological pain states, but whether changes in serotonergic mechanisms are pro or anti-nociceptive are debated. We used a combination of optogenetics and fiber photometry to examine these mechanisms more closely. We find that optogenetic activation of RVM serotonergic afferents in the spinal cord of naïve mice produces mechanical hypersensitivity and conditioned place aversion. Neuropathic pain, produced by chronic constriction injury of the infraorbital nerve (CCI-ION), evoked a tonic increase in serotonin concentrations within the spinal trigeminal nucleus caudalis (SpVc), measured with liquid chromatography-tandem mass spectroscopy (LC-MS/MS). By contract, CCI-ION had no effect on the phasic serotonin transients in SpVc, evoked by noxious pinch, and measured with fiber photometry of a serotonin sensor. These findings suggest that serotonin release in the spinal cord is pronociceptive and that an increase is sustained serotonin signaling, rather than phasic or event driven increases, potentiate nociception in models of chronic pain.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures


References
-
- Allers KA, Sharp T (2003) Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience, 122:193–204. - PubMed
-
- Andersen E, Dafny N (1983) An ascending serotonergic pain modulation pathway from the dorsal raphe nucleus to the parafascicularis nucleus of the thalamus. Brain Res, 269:57–67. - PubMed
-
- Bannister K, Sachau J, Baron R, Dickenson AH (2020) Neuropathic Pain: Mechanism-Based Therapeutics. Annu Rev Pharmacol Toxicol, 60:257–274. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
