This is a preprint.
The Genetic Landscape of a Metabolic Interaction
- PMID: 37645784
- PMCID: PMC10461916
- DOI: 10.1101/2023.05.28.542639
The Genetic Landscape of a Metabolic Interaction
Update in
-
The genetic landscape of a metabolic interaction.Nat Commun. 2024 Apr 18;15(1):3351. doi: 10.1038/s41467-024-47671-0. Nat Commun. 2024. PMID: 38637543 Free PMC article.
Abstract
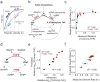
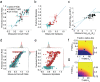
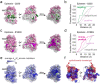
Enzyme abundance, catalytic activity, and ultimately sequence are all shaped by the need of growing cells to maintain metabolic flux while minimizing accumulation of deleterious intermediates. While much prior work has explored the constraints on protein sequence and evolution induced by physical protein-protein interactions, the sequence-level constraints emerging from non-binding functional interactions in metabolism remain unclear. To quantify how variation in the activity of one enzyme constrains the biochemical parameters and sequence of another, we focused on dihydrofolate reductase (DHFR) and thymidylate synthase (TYMS), a pair of enzymes catalyzing consecutive reactions in folate metabolism. We used deep mutational scanning to quantify the growth rate effect of 2,696 DHFR single mutations in 3 TYMS backgrounds under conditions selected to emphasize biochemical epistasis. Our data are well-described by a relatively simple enzyme velocity to growth rate model that quantifies how metabolic context tunes enzyme mutational tolerance. Together our results reveal the structural distribution of epistasis in a metabolic enzyme and establish a foundation for the design of multi-enzyme systems.
Keywords: DHFR; TYMS; deep mutational scan; dihydrofolate reductase; epistasis; genetic interaction; genotype-to-phenotype; thymidylate synthase.
Conflict of interest statement
COMPETING INTERESTS The authors have no competing interests to declare.
Figures




References
-
- Kemble H. E. et al. Flux, toxicity and protein expression costs shape genetic interaction in a metabolic pathway. http://biorxiv.org/lookup/doi/10.1101/362327 (2018) doi: 10.1101/362327. - DOI - PMC - PubMed
-
- Dekel E. & Alon U. Optimality and evolutionary tuning of the expression level of a protein. Nature 436, 588–592 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
