This is a preprint.
Structure of C. elegans TMC-2 complex suggests roles of lipid-mediated subunit contacts in mechanosensory transduction
- PMID: 37645790
- PMCID: PMC10462014
- DOI: 10.1101/2023.08.16.553618
Structure of C. elegans TMC-2 complex suggests roles of lipid-mediated subunit contacts in mechanosensory transduction
Update in
-
The structure of the Caenorhabditis elegans TMC-2 complex suggests roles of lipid-mediated subunit contacts in mechanosensory transduction.Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2314096121. doi: 10.1073/pnas.2314096121. Epub 2024 Feb 14. Proc Natl Acad Sci U S A. 2024. PMID: 38354260 Free PMC article.
Abstract
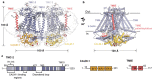
Mechanotransduction is the process by which a mechanical force, such as touch, is converted into an electrical signal. Transmembrane channel-like (TMC) proteins are an evolutionarily-conserved family of ion channels whose function has been linked to a variety of mechanosensory processes, including hearing and balance sensation in vertebrates and locomotion in Drosophila. The molecular features that tune homologous TMC ion channel complexes to diverse mechanical stimuli are unknown. Caenorhabditis elegans express two TMC homologs, TMC-1 and TMC-2, both of which are the likely pore-forming subunits of mechanosensitive ion channels but differ in their expression pattern and functional role in the worm. Here we present the single particle cryo-electron microscopy structure of the native TMC-2 complex isolated from C. elegans. The complex is composed of two copies each of the pore-forming TMC-2 subunit, the calcium and integrin binding protein CALM-1 and the transmembrane inner ear protein TMIE. Comparison of the TMC-2 complex to the recently published cryo-EM structure of the C. elegans TMC-1 complex reveals differences in subunit composition and highlights conserved protein-lipid interactions, as well as other structural features, that together suggest a mechanism for TMC-mediated mechanosensory transduction.
Conflict of interest statement
Competing Interest Statement: The authors declare no competing interests.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
