This is a preprint.
Increasing the accuracy of single-molecule data analysis using tMAVEN
- PMID: 37645812
- PMCID: PMC10462008
- DOI: 10.1101/2023.08.15.553409
Increasing the accuracy of single-molecule data analysis using tMAVEN
Update in
-
Increasing the accuracy of single-molecule data analysis using tMAVEN.Biophys J. 2024 Sep 3;123(17):2765-2780. doi: 10.1016/j.bpj.2024.01.022. Epub 2024 Jan 24. Biophys J. 2024. PMID: 38268189 Free PMC article.
Abstract
Time-dependent single-molecule experiments contain rich kinetic information about the functional dynamics of biomolecules. A key step in extracting this information is the application of kinetic models, such as hidden Markov models (HMMs), which characterize the molecular mechanism governing the experimental system. Unfortunately, researchers rarely know the physico-chemical details of this molecular mechanism a priori, which raises questions about how to select the most appropriate kinetic model for a given single-molecule dataset and what consequences arise if the wrong model is chosen. To address these questions, we have developed and used time-series Modeling, Analysis, and Visualization ENvironment (tMAVEN), a comprehensive, open-source, and extensible software platform. tMAVEN can perform each step of the single-molecule analysis pipeline, from pre-processing to kinetic modeling to plotting, and has been designed to enable the analysis of a single-molecule dataset with multiple types of kinetic models. Using tMAVEN, we have systematically investigated mismatches between kinetic models and molecular mechanisms by analyzing simulated examples of prototypical single-molecule datasets exhibiting common experimental complications, such as molecular heterogeneity, with a series of different types of HMMs. Our results show that no single kinetic modeling strategy is mathematically appropriate for all experimental contexts. Indeed, HMMs only correctly capture the underlying molecular mechanism in the simplest of cases. As such, researchers must modify HMMs using physico-chemical principles to avoid the risk of missing the significant biological and biophysical insights into molecular heterogeneity that their experiments provide. By enabling the facile, side-by-side application of multiple types of kinetic models to individual single-molecule datasets, tMAVEN allows researchers to carefully tailor their modeling approach to match the complexity of the underlying biomolecular dynamics and increase the accuracy of their single-molecule data analyses.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

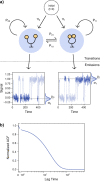



References
-
- Bustamante C., Bryant Z., and Smith S.B.. 2003. Ten years of tension: single-molecule DNA mechanics. Nature. 421:423–427. - PubMed
-
- MacDougall D.D., Fei J., and Gonzalez R.L.. 2011. Single-Molecule Fluorescence Resonance Energy Transfer Investigations of Ribosome-Catalyzed Protein Synthesis. In: Frank J, editor. Molecular Machines in Biology. Cambridge: Cambridge University Press. pp. 93–116.
-
- Du C., and Kou S.C.. 2020. Statistical Methodology in Single-Molecule Experiments. Statistical Science. 35:75–91.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
