This is a preprint.
A non-canonical mechanism of GPCR activation
- PMID: 37645874
- PMCID: PMC10462065
- DOI: 10.1101/2023.08.14.553154
A non-canonical mechanism of GPCR activation
Update in
-
A non-canonical mechanism of GPCR activation.Nat Commun. 2024 Nov 16;15(1):9938. doi: 10.1038/s41467-024-54103-6. Nat Commun. 2024. PMID: 39550377 Free PMC article.
Abstract
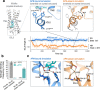
The goal of designing safer, more effective drugs has led to tremendous interest in molecular mechanisms through which ligands can precisely manipulate signaling of G-protein-coupled receptors (GPCRs), the largest class of drug targets. Decades of research have led to the widely accepted view that all agonists-ligands that trigger GPCR activation-function by causing rearrangement of the GPCR's transmembrane helices, opening an intracellular pocket for binding of transducer proteins. Here we demonstrate that certain agonists instead trigger activation of free fatty acid receptor 1 by directly rearranging an intracellular loop that interacts with transducers. We validate the predictions of our atomic-level simulations by targeted mutagenesis; specific mutations which disrupt interactions with the intracellular loop convert these agonists into inverse agonists. Further analysis suggests that allosteric ligands could regulate signaling of many other GPCRs via a similar mechanism, offering rich possibilities for precise control of pharmaceutically important targets.
Conflict of interest statement
Competing Financial Interests A.K., S.S., J.D.S., J.L., S.M.S., J.M.J., A.M.W. are current or past employees of Merck Research Laboratories.
Figures




References
-
- Chan H. C. S., Li Y., Dahoun T., Vogel H. & Yuan S. New Binding Sites, New Opportunities for GPCR Drug Discovery. Trends Biochem. Sci. 44, 312–330 (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
