Functions and mechanisms of lactylation in carcinogenesis and immunosuppression
- PMID: 37646027
- PMCID: PMC10461103
- DOI: 10.3389/fimmu.2023.1253064
Functions and mechanisms of lactylation in carcinogenesis and immunosuppression
Abstract
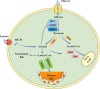
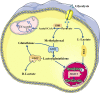
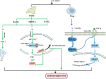
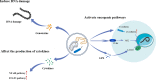
As critical executors regulating many cellular operations, proteins determine whether living activities can be performed in an orderly and efficient manner. Precursor proteins are inert and must be modified posttranslationally to enable a wide range of protein types and functions. Protein posttranslational modifications (PTMs) are well recognized as being directly associated with carcinogenesis and immune modulation and have emerged as important targets for cancer detection and treatment. Lactylation (Kla), a novel PTM associated with cellular metabolism found in a wide range of cells, interacts with both histone and nonhistone proteins. Unlike other epigenetic changes, Kla has been linked to poor tumor prognosis in all current studies. Histone Kla can affect gene expression in tumors and immunological cells, thereby promoting malignancy and immunosuppression. Nonhistone proteins can also regulate tumor progression and treatment resistance through Kla. In this review, we aimed to summarize the role of Kla in the onset and progression of cancers, metabolic reprogramming, immunosuppression, and intestinal flora regulation to identify new molecular targets for cancer therapy and provide a new direction for combined targeted therapy and immunotherapy.
Keywords: Warburg effect; immunosuppression; lactylation (Kla); metabolic reprogramming; tumor microenvironment (TME).
Copyright © 2023 Su, Zheng, Bian, Chang, Bao, Yu, Xin and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in toll-like receptor- and inflammasome-mediated inflammation via gpr81-mediated suppression of innate immunity. Gastroenterology (2014) 146(7):1763–74. doi: 10.1053/j.gastro.2014.03.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

