CD62L as target receptor for specific gene delivery into less differentiated human T lymphocytes
- PMID: 37646032
- PMCID: PMC10461316
- DOI: 10.3389/fimmu.2023.1183698
CD62L as target receptor for specific gene delivery into less differentiated human T lymphocytes
Abstract
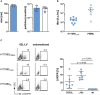
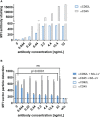
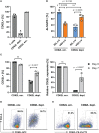
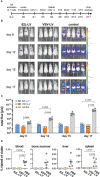
Chimeric antigen receptor (CAR)-expressing T cells are a complex and heterogeneous gene therapy product with variable phenotype compositions. A higher proportion of less differentiated CAR T cells is usually associated with improved antitumoral function and persistence. We describe in this study a novel receptor-targeted lentiviral vector (LV) named 62L-LV that preferentially transduces less differentiated T cells marked by the L-selectin receptor CD62L, with transduction rates of up to 70% of CD4+ and 50% of CD8+ primary T cells. Remarkably, higher amounts of less differentiated T cells are transduced and preserved upon long-term cultivation using 62L-LV compared to VSV-LV. Interestingly, shed CD62L neither altered the binding of 62L-LV particles to T cells nor impacted their transduction. The incubation of 2 days of activated T lymphocytes with 62L-LV or VSV-LV for only 24 hours was sufficient to generate CAR T cells that controlled tumor growth in a leukemia tumor mouse model. The data proved that potent CAR T cells can be generated by short-term ex vivo exposure of primary cells to LVs. As a first vector type that preferentially transduces less differentiated T lymphocytes, 62L-LV has the potential to circumvent cumbersome selections of T cell subtypes and offers substantial shortening of the CAR T cell manufacturing process.
Keywords: CAR T cells; L-selectin; LV; chimeric antigen receptor; naïve T lymphocytes; receptor-targeted viral vectors; ΔLNGFR.
Copyright © 2023 Kapitza, Ho, Kerzel, Frank, Thalheimer, Jamali, Schaser, Buchholz and Hartmann.
Conflict of interest statement
CB, JH, FT, LK, and TS are listed as inventors on a patent describing 62L-LV. Author TS is a full-time employee of the company Miltenyi Biotec GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Optimizing lentiviral vector formulation conditions for efficient ex vivo transduction of primary human T cells in chimeric antigen receptor T-cell manufacturing.Cytotherapy. 2024 Sep;26(9):1084-1094. doi: 10.1016/j.jcyt.2024.04.002. Epub 2024 Apr 7. Cytotherapy. 2024. PMID: 38661611
-
Highly Efficient and Selective CAR-Gene Transfer Using CD4- and CD8-Targeted Lentiviral Vectors.Mol Ther Methods Clin Dev. 2019 Mar 16;13:371-379. doi: 10.1016/j.omtm.2019.03.003. eCollection 2019 Jun 14. Mol Ther Methods Clin Dev. 2019. PMID: 30997367 Free PMC article.
-
In Vivo Generation of CAR T Cells Selectively in Human CD4+ Lymphocytes.Mol Ther. 2020 Aug 5;28(8):1783-1794. doi: 10.1016/j.ymthe.2020.05.005. Epub 2020 May 16. Mol Ther. 2020. PMID: 32485137 Free PMC article.
-
CAR Gene Delivery by T-cell Targeted Lentiviral Vectors is Enhanced by Rapamycin Induced Reduction of Antiviral Mechanisms.Adv Sci (Weinh). 2023 Dec;10(35):e2302992. doi: 10.1002/advs.202302992. Epub 2023 Oct 30. Adv Sci (Weinh). 2023. PMID: 37904721 Free PMC article.
-
Surface-Engineered Lentiviral Vectors for Selective Gene Transfer into Subtypes of Lymphocytes.Mol Ther Methods Clin Dev. 2018 Oct 17;12:19-31. doi: 10.1016/j.omtm.2018.10.006. eCollection 2019 Mar 15. Mol Ther Methods Clin Dev. 2018. PMID: 30417026 Free PMC article. Review.
Cited by
-
Optimizing viral transduction in immune cell therapy manufacturing: key process design considerations.J Transl Med. 2025 May 2;23(1):501. doi: 10.1186/s12967-025-06524-0. J Transl Med. 2025. PMID: 40316943 Free PMC article. Review.
References
-
- Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. . Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA (2009) 106:17469–74. doi: 10.1073/pnas.0907448106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

